Establishing Age-Associated Normative Ranges of the Cerebral 18F-FDG Uptake Ratio in Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -
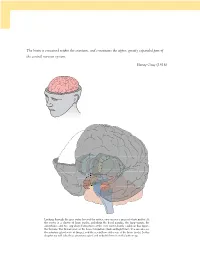
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Diverging Patterns of Atrophy and Hypometabolism in Syndromic
Simultaneous PET-MRI studies of the concordance of atrophy and hypometabolism in syndromic variants of Alzheimer’s disease and frontotemporal dementia: an extended case series *Kuven K Moodley1, *Daniela Perani2, Ludovico Minati1,3, Pasquale Anthony Della Rosa4, Frank Pennycook5, John C Dickson6, Anna Barnes6, Valeria Elisa Contarino7, Sofia Michopoulou7, Ludovico D’Incerti7, Catriona Good8, Federico Fallanca2, Emilia Giovanna Vanoli2, Peter J Ell6, and Dennis Chan1 *These authors equally contributed to the work 1Brighton and Sussex Medical School, Falmer, UK 2 Vita-Salute San Raffaele University, Nuclear Medicine Unit San Raffaele Hospital, Division of Neuroscience IRCCS San Raffaele, Milano, Italy 3Scientific Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano, Italy 4Institute of Molecular Bio-imaging and Physiology, National Research Council, Milano, Italy 5Open University, Milton Keynes, UK 6Institute of Nuclear Medicine, University College London, London, UK 7Neuroradiology Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano, Italy 8Hurstwood Park Neurosciences Centre, West Sussex, UK Corresponding Author: Dr Dennis Chan Herchel Smith Building for Brain and Mind Sciences Department of Clinical Neurosciences University of Cambridge Forvie Site, Robinson Way Cambridge CB2 0SZ Email: [email protected] Word count Abstract 278 Main Text 4969 Tables 1 Figures 6 Supplementary Figures 6 1 Abstract Aims. The primary objective was to determine the concordance of brain atrophy and hypometabolism in six syndromic variants of Alzheimer’s disease (AD) and frontotemporal dementia spectrum (FTD). A second objective was to determine the effect of image analysis methods on identification of atrophy and hypometabolism by comparing the changes observed through qualitative rating with those detected by quantitative methods. -

Brown Medical School Biomed 370 the Brain and Human Behavior
Brown Medical School Biomed 370 The Brain and Human Behavior The Brain and Human Behavior Biomed 370 A first year, second semester course sponsored by the Department of Psychiatry and Human Behavior Course Directors: Robert Boland, M.D. 455-6417 (office), 455-6497 (fax) [email protected] Stephen Salloway, M.D., M.S. 455-6403 (office), 455-6405 (fax) [email protected] Web site available through WebCT Teaching Assistants: Nancy Brim ([email protected]) Marisa Kastoff ([email protected]) Stan Pelosi ([email protected]) Grace Farris ([email protected]) Table of Contents. Overall Course Objectives.....................................................................4 Section 1. Basic Principles. ...................................................................7 Chapter 1. Limbic System Anatomy........................................................8 Chapter 2. Frontal Lobe Function And Dysfunction..............................13 Chapter 3. Clinical Neurochemistry......................................................19 Chapter 4. The Neurobiology Of Memory............................................36 Chapter 5. The Control Of Feeding Behavior .......................................46 Chapter 6. Principles Of Pharmacology................................................51 Chapter 7. Principles Of Neuroimaging.................................................55 Chapter 8. The Mental Status Examination...........................................74 Section 2. The Clinical Disorders.......................................................89 -

SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This
12/19/2018 11:00 AM FOUNDATIONAL LEARNING SYSTEM 092892-181219 © Johnson & Johnson Servicesv Inc. 2018 All rights reserved. 1 SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This module will strengthen your understanding of basic neuroanatomy, neurovasculature, and functional roles of specific brain regions. 1 12/19/2018 11:00 AM Lesson 1: Introduction to the Brain The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. In this lesson, we’ll discuss the principle brain regions, layers of the brain, and lobes of the brain, as well as common terms used to orient neuroanatomical discussions. 2 SAY: The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. (Purves 2012/p717/para1) In this lesson, we’ll explore these organizational layers by discussing the principle brain regions, layers of the brain, and lobes of the brain. We’ll also discuss the terms used by scientists and healthcare providers to orient neuroanatomical discussions. 2 12/19/2018 11:00 AM Lesson 1: Learning Objectives • Define terms used to specify neuroanatomical locations • Recall the 4 principle regions of the brain • Identify the 3 layers of the brain and their relative location • Match each of the 4 lobes of the brain with their respective functions 3 SAY: Please take a moment to review the learning objectives for this lesson. 3 12/19/2018 11:00 AM Directional Terms Used in Anatomy 4 SAY: Specific directional terms are used when specifying the location of a structure or area of the brain. -

Tutorial: Memory and Memory Problems
Tutorial: Memory and Memory Problems WHAT IS MEMORY? Memory is one of the central components of human cognition, including the ability to take in information, process it, store it, and subsequently retrieve it when necessary. Thus the core processes of memory are encoding, storage, and retrieval: Encoding: Processing information, organizing it, and marking it for storage Storage: Holding information over time in what is ideally an organized storage system Retrieval: Calling stored information to consciousness Following TBI, both encoding and retrieval can be significantly impaired. However, storage (i.e., keeping information in storage after it has been effectively processed) is often relatively spared. Therefore, if information can be effectively processed and encoded, it is more likely to be retained, even though it may be difficult to retrieve. Video Illustration of Types of Retrieval Memory Processes and Systems Authorities on memory typically explain human memory by drawing a variety of distinctions among different types or aspects of memory. Understanding many of these distinctions is important for staff and family members working with students with memory and learning impairments. Voluntary and involuntary memory: Encoding: Encoding of information for storage in long-term memory can be either involuntary (incidental, implicit) or voluntary (effortful, deliberate, strategic). Involuntary encoding occurs when the goal of the activity is something other than memory or learning, and memory occurs as a bi-product. For example, a young student may remember the names of geometric figures not by trying to memorize them, but rather because he was involved in an art project in which it was important to process the names of the geometric figures in order to complete the project. -

Memory and the Brain
CHAPTER 2 Memory and the Brain T he word brain really means different things to different people. In everyday usage, the word brain is nearly synonymous with the word mind. We say that we have something “in our brain that we cannot get out,” meaning we have been thinking about something. You call someone a “brain” if you think that his or her intelligence is that person’s chief char - acteristic. However, underlying this metaphor is the certainty that the brain is the biologi - cal organ responsible for thinking, memory, reasoning, and language. In this chapter, we will explore the science of how the brain produces memory. For a neurosurgeon, the brain is a mass of soft tissue inside the head that has to be han - dled very carefully when damaged. The brain itself has no pain receptors, so neurosurgeons are less concerned about anesthesia than other doctors. However, the brain is surrounded and infused with millions of blood vessels, so surgeons must be very careful when probing around the brain, lest they accidentally induce a hemorrhage. Neurosurgeons understand the critical nature of the human brain for what it is to be human, yet for a surgeon, its iden - tity is a biological tissue. For a cognitive neuroscientist, the brain is a complex assortment of separate areas and regions, each of which has its own unique function. For example, the frontal regions are for planning, thinking, and monitoring, while the back of the brain processes vision. Viewed this way, the brain is not really one organ but many dozens of distinct regions each with its own appearance, its own micro-anatomy, and its own function. -

Biblical Texts Provide Several Examples to Support the Notion of the Earth Standing Still While Other Bodies Move Around It, As in the Story of Joshua
Science and Religion Understanding the Issues Nancy Morvillo A John Wiley & Sons, Ltd., Publication Science and Religion Science and Religion Understanding the Issues Nancy Morvillo A John Wiley & Sons, Ltd., Publication This edition first published 2010 © 2010 Nancy Morvillo Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing program has been merged with Wiley’s global Scientific, Technical, and Medical business to form Wiley-Blackwell. Registered Office John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom Editorial Offices 350 Main Street, Malden, MA 02148-5020, USA 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK For details of our global editorial offices, for customer services, and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell. The right of Nancy Morvillo to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. Designations used by companies to distinguish their products are often claimed as trademarks. -

The Criminal Brain: Frontal Lobe Dysfunction Evidence in Capital Proceedings, 16 Cap
Capital Defense Journal Volume 16 | Issue 2 Article 4 Spring 3-1-2004 The rC iminal Brain: Frontal Lobe Dysfunction Evidence in Capital Proceedings Jessie A. Seiden Follow this and additional works at: https://scholarlycommons.law.wlu.edu/wlucdj Part of the Law Enforcement and Corrections Commons Recommended Citation Jessie A. Seiden, The Criminal Brain: Frontal Lobe Dysfunction Evidence in Capital Proceedings, 16 Cap. DEF J. 395 (2004). Available at: https://scholarlycommons.law.wlu.edu/wlucdj/vol16/iss2/4 This Article is brought to you for free and open access by the Law School Journals at Washington & Lee University School of Law Scholarly Commons. It has been accepted for inclusion in Capital Defense Journal by an authorized editor of Washington & Lee University School of Law Scholarly Commons. For more information, please contact [email protected]. The Criminal Brain: Frontal Lobe Dysfunction Evidence in Capital Proceedings Jessie A. Seiden* L Inxaion Something was wrong with the 40-year-old schoolteacher from Virginia.' From all outward appearances he seemed to be living a completely normal life.2 What others did not know was that he had begun collecting child pornography, soliciting prostitutes, and making sexual advances toward his stepdaughter.3 Eventually his wife found out and he was ordered either to complete a Sexaholics Anonymous program or face jail time.4 Despite the threat of jailtime, he failed the program because he could not stop himself from asking the other members for sexual favors.' The night before he was scheduled for sentencing, the man went to a hospital and complained of severe headaches.' During his hospitalization the man continued acting out and even propositioned nurses.7 Doctors performed a neurologic exam and discovered a large brain tumor displacing his orbitofrontal lobe.8 After the tumor was removed, the deviant urges subsided and he was allowed to return home.' * J.D. -

The Brain and Behaviour
www.alzheimers.org.uk The brain and behaviour Alzheimer's disease is a degenerative condition associated with a progressive loss of nerve cells or neurons. The disease gradually pervades most areas of the brain. However, the evolving pattern of damage can vary greatly between different individuals. As a result, each person with the disease may have a complex set of difficulties and experiences which are peculiar to them. This information sheet explains which areas of the brain are largely responsible for which skills and abilities, and how the changes in behaviour, memory and thought we see in people with Alzheimer's may be a direct result of the way the disease has affected the brain. The brain consists of three main sections: the hindbrain, the midbrain and the forebrain (see figure 1). The hindbrain and midbrain are mainly concerned with basic life support functions such as blood pressure and respiration. In contrast, the forebrain is responsible for the majority of higher brain functions such as memory and language. The cerebral cortex The most important part of the forebrain is the cerebral cortex, a thin shell covering the surface of the forebrain. This thin layer is tightly crumpled and folded to increase its surface area. As a result the cortex alone contains an astonishing ten thousand million brain cells (the 'grey matter'). Beneath this densely packed cortex lie bundles of fibres (the 'white matter') which transport information around the cortex and to other brain regions (see figure 3). The lobes Page 1 www.alzheimers.org.uk To help us describe the different parts of the brain, the forebrain has been divided up into four sections or 'lobes' which are shown in figure 2. -
Part I: Lobes, the Cerebral Cortex, and Cortical Regions of the Brain
The Human Brain Master Watermark Image: http://williamcalvin.com/BrainForAllSeasons/img/bonoboLH-humanLH-viaTWD.gif Part I: Lobes, the Cerebral Cortex, and Cortical Regions of the Brain Objectives: • Students will be able to describe the general structure of the Cerebrum and Cerebral Cortex. • Students will be able to identify the Cerebrum, the Lobes of the Brain, the Cerebral Cortex, and its major regions/divisions. • Students will be able to describe the primary functions of the Lobes and the Cortical Regions of the Brain. Cerebrum -The largest division of the brain. It is divided into two hemispheres, each of which is divided into four lobes. Cerebrum Cerebrum Cerebellum http://williamcalvin.com/BrainForAllSeasons/img/bonoboLH-humanLH-viaTWD.gif Cerebral Cortex - The outermost layer of gray matter making up the superficial aspect of the cerebrum. Cerebral Cortex Cerebral Cortex http://www.bioon.com/book/biology/whole/image/1/1-6.tif.jpg Cerebral Features: • Gyri – Elevated ridges “winding” around the brain. • Sulci – Small grooves dividing the gyri – Central Sulcus – Divides the Frontal Lobe from the Parietal Lobe • Fissures – Deep grooves, generally dividing large regions/lobes of the brain – Longitudinal Fissure – Divides the two Cerebral Hemispheres – Transverse Fissure – Separates the Cerebrum from the Cerebellum – Sylvian/Lateral Fissure – Divides the Temporal Lobe from the Frontal and Parietal Lobes Gyri (ridge) Sulci (groove) Fissure (deep groove) http://williamcalvin.com/BrainForAllSeasons/img/bonoboLH-humanLH-viaTWD.gif Specific Sulci/Fissures: Central Sulcus Longitudinal Fissure Sylvian/Lateral Fissure Transverse Fissure http://www.bioon.com/book/biology/whole/image/1/1-8.tif.jpg http://www.dalbsoutss.eq.edu.au/Sheepbrains_Me/human_brain.gif Lobes of the Brain (4) • Frontal • Parietal • Occipital • Temporal http://www.bioon.com/book/biology/whole/image/1/1-8.tif.jpg * Note: Occasionally, the Insula is considered the fifth lobe. -

A Brain Primer— Major Structures and Their Functions
C HAPTER 1 A Brain Primer— Major Structures and Their Functions Education is discovering the brain and that’s about the best news there could be . anyone who does not have a thor- ough, holistic grasp of the brain’s architecture, purposes, and main ways of operating is as far behind the times as an auto- mobile designer without a full understanding of engines. —Leslie Hart (1983) • Have you ever wondered how the brain works? • Have you ever wondered what the different parts of the brain do? • Have you ever wondered why some see effort as futile and others see it as imperative? The brain is a complex organ whose relationship with learn- ing is undeniable. The primary charge of an instructional leader is to increase learning. Analyze the learning organ and begin to understand how we learn. To develop understanding, it is criti- cal to have anchors of information from which to build upon. 1 2 THE INSTRUCTIONAL LEADER AND THE BRAIN This chapter will act as that anchor. Because the remaining chapters in the book may assume knowledge of this chapter, it will serve as a foundation for greater comprehension throughout the remainder of the book. This chapter will discuss the major struc- tures and functions of the brain, what the brain looks and feels like, and how those structures differ in their functions. Readers will explore deep inside the brain to discover the mysteries of this miraculous organ. Does not understanding how the brain works preclude being an effective instructional leader? Probably not, especially for those who have already enjoyed years of success as an instruc- tional leader.