Alkanes-1 [Compatibility Mode]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Defining Potential Chemical Peaks and Management Options
PROJECT NO. 4991 Defining Potential Chemical Peaks and Management Options Defining Potential Chemical Peaks and Management Options Prepared by: Jean Debroux Kennedy Jenks Consultants Megan H. Plumlee Orange County Water District Shane Trussell Trussell Technologies, Inc. Co-sponsored by: California State Water Resources Control Board 2021 The Water Research Foundation (WRF) is a nonprofit (501c3) organization which provides a unified source for One Water research and a strong presence in relationships with partner organizations, government and regulatory agencies, and Congress. The foundation conducts research in all areas of drinking water, wastewater, stormwater, and water reuse. The Water Research Foundation’s research portfolio is valued at over $700 million. WRF plays an important role in the translation and dissemination of applied research, technology demonstration, and education, through creation of research-based educational tools and technology exchange opportunities. WRF serves as a leader and model for collaboration across the water industry and its materials are used to inform policymakers and the public on the science, economic value, and environmental benefits of using and recovering resources found in water, as well as the feasibility of implementing new technologies. For more information, contact: The Water Research Foundation 1199 North Fairfax Street, Suite 900 6666 West Quincy Avenue Alexandria, VA 22314-1445 Denver, Colorado 80235-3098 www.waterrf.org P 571.384.2100 P 303.347.6100 [email protected] ©Copyright 2021 by The Water Research Foundation. All rights reserved. Permission to copy must be obtained from The Water Research Foundation. WRF ISBN: 978-1-60573-555-9 WRF Project Number: 4991 This report was prepared by the organization(s) named below as an account of work sponsored by The Water Research Foundation. -

United States Patent 19 11 3,982,028 Bellingham (45) Sept
United States Patent 19 11 3,982,028 Bellingham (45) Sept. 21, 1976 (54) PRESERVATIVES FOR FORAGE, HAY, 3,806,600 4/1974 Lapore................................ 424/317 GRAN AND ANIMAL FEEDS FOREIGN PATENTS OR APPLICATIONS 75) Inventor: Francis Bellingham, 1,276,677 6/1972 United Kingdom....................... 99/8 Stockton-on-Tees, England OTHER PUBLICATIONS (73) Assignee: Imperial Chemical Industries Condensed Chemical Dictionary - Hawley, 8th Ed., Limited, London, England Van Nostrand, May 1971, p. 481. 22 Filed: Sept. 13, 1973 Technical News Letter, Ministry of Agriculture, 1 1/68, 10/4/68. 21 Appl. No.: 396,666 H. D. Young, Chem. Abs., v.24, 1930, p. 3595. (30) Foreign Application Priority Data Primary Examiner-Norman Yudkoff Sept. 4, 1972 United Kingdom............... 2002 Attorney,Assistant ExaminerAgent, or Firm-Cushman,Hiram H. Bernstein Darby & 52 U.S. Cl................................... 426/69; 424/317; Cushman 426/807; 426/335; 426/532; 426/636; 426/635 57 ABSTRACT (51 Int. Cl.'............................................ A23K 1100 Feedstuffs and silage for ruminants contain mixtures 58) Field of Search ........... 426/15, 227, 310, 221, of isobutyraldehyde (IBD) with mineral or fatty acids, 426/331, 335, 210, 69,807, 635, 636,532; formalin, trioxane, or urea plus chlorine, derivatives of 424/317; 252/.407; 260/526 IBD selected from isobutanol (alone or in admixture with isobutyric acid or salts thereof), isobutyl halides 56 References Cited or esters of fatty acids, isobutyrates, di-isobutyrates, UNITED STATES PATENTS and chlorinated isobutylidene diurea. 3,642,488 2/1972 Watchorn............................. 426/69 7 Claims, No Drawings 3,982,028 2 Table 1-continued PRESERVATIVES FOR FORAGE, HAY, GRAIN Additives which showed preservative properties in AND ANIMAL FEEDS compound animal feeds 5 Treatment The present invention relates to substances suitable Additive level 7 ww for feeding to animals (particularly to ruminants) in 4 IBDisobutyric acid mixtures (e.g. -

Toxicity Review for Dibutyl Adipate (DBA) and Di-Isobutyl Adipate (Diba)”1 June 2019
CPSC Staff Statement on University of Cincinnati Report “Toxicity Review for Dibutyl Adipate (DBA) and Di-isobutyl Adipate (DiBA)”1 June 2019 The U.S. Consumer Product Safety Commission (CPSC) contracted with the University of Cincinnati to conduct toxicology assessments for nine dialkyl o-phthalate (o-DAP) substitutes: phenyl esters of C10-C18 alkylsulfonic acid esters (ASE); glycerides, castor-oil-mono-, hydrogenated, acetates (COMGHA); dibutyl adipate (DBA) and di-isobutyl adipate (DiBA); di (2-ethylhexyl) sebacate (DEHS)/dioctyl sebacate (DOS); a mixture of 98% di-2-ethylhexyl terephthalate (DEHT) and 2% 2-ethylhexyl methyl terephthalate (2-EHMT); dibutyl sebacate (DBS); diisononyl adipate (DINA); epoxidized soybean oil (ESBO); and tributyl citrate (TBC). The reports will be used to inform staff’s assessment of products that may contain these compounds and is the first step in the risk assessment process. CPSC staff assesses a product’s potential health effects to consumers under the Federal Hazardous Substances Act (FHSA). The FHSA is risk-based. To be considered a “hazardous substance” under the FHSA, a consumer product must satisfy a two-part definition. First, it must be “toxic” under the FHSA, or present one of the other hazards enumerated in the statute. Second, it must have the potential to cause “substantial personal injury or substantial illness during or as a proximate result of any customary or reasonably foreseeable handling or use.” Therefore, exposure and risk must be considered in addition to toxicity when assessing potential hazards of products under the FHSA. The first step in the risk assessment process is hazard identification, which consists of a review of the available toxicity data for the chemical. -

Aluminum Chemical Compatibility Chart From
ver 16-Jan-2019 Aluminum Chemical Compatibility Chart Chemical Chemical Acetaldehyde (ethanal) B Alcohols: Fatty B Acetamide (ethanamide) A Alcohols: Hexyl (1-hexanol) A Acetate Solvents A Alcohols: Isobutyl (2-methyl-1-propanol) B Acetic Acid C Alcohols: Isopropyl (2-propanol) B Acetic Acid, 10% C Alcohols: Methyl (methanol) A Acetic Acid, 20% C Alcohols: Octyl (caprylic alcohol) A Acetic Acid, 30% C Alcohols: Propyl (propanol) A Acetic Acid, 50% C Allyl Alcohol B Acetic Acid, 80% C Allyl Bromide D Acetic Acid, aerated C Allyl Chloride D Acetic Acid, air free C Alum (aluminum potassium sulfate) C Acetic Acid, crude C Aluminum Acetate A Acetic Acid, glacial C Aluminum Chloride D Acetic Acid Vapors B Aluminum Chloride, 20% D Acetic Anhydride A Aluminum Chlorohydroxide D Acetone A Aluminum Fluoride B Acetone Cyanohydrin B Aluminum Hydroxide B Acetonitrile (methyl cyanide) A Aluminum Nitrate D Acetophenone (acetyl benzene) B Aluminum Oxalate B Acetyl Acetone D Aluminum Potassium Sulfate, 10% C Acetyl Chloride, dry D Aluminum Potassium Sulfate, 100% C Acetyl Salicylic Acid (aspirin) D Aluminum Sulfate B Acetylene A Alums A Acetylene Tetrabromide D Amines B Acrolein (acrylaldehyde) B Ammonia, 10% (ammonium hydroxide) A Acrylonitrile (vinyl cyanide) B Ammonia Nitrate C Adipic Acid A Ammonia, anhydrous B Alcohols: Allyl B Ammonia, liquid A Alcohols: Amyl (1-pentanol) B Ammonium Acetate A Alcohols: Benzyl (phenol carbinol) B Ammonium Bicarbonate B Alcohols: Butyl (butanol) B Ammonium Bifluoride B Alcohols: Capryl (octanol) A Ammonium Bromide, -

Solicit Proposals for a Firm to Provide a Chemical Tracking Software System at the Collge of New Jersey
Number: AB150031 Date Issued: March 15, 2015 Purchasing Contact: Roselle Horodeski Phone: (609) 771-2495 Email: [email protected] Requesting Department: Administrative and Environmental Services Fiscal Year: 2015 _______________________________________________ Proposals will be due on Wednesday, April 22, 2015 at 2 p.m. __________________________________________________________ Important: This proposal must be received at or before the opening time and date stated above. Late proposals will not be accepted. Return proposal to: The College of New Jersey Office of Finance & Business Services, Purchasing Dept. Administrative Services Building, Room 201 2000 Pennington Road P.O. Box 7718 Ewing, New Jersey 08628-0718 609-771-2495 _______________________________________________________________________________________________________________ PURPOSE AND INTENT OF PROPOSAL: Solicit proposals for a firm to provide a Chemical Tracking Software System at The Collge of New Jersey. INSTRUCTIONS TO BIDDERS FOR COMPLETING THIS PROPOSAL 1. Read the entire proposal, including all terms and conditions and specifications. 2. All prices must be typed or written in ink. Any corrections, erasures or other forms of alteration to unit and/or total prices must be initialed by the bidder. 3. THIS PROPOSAL IS TO BE SIGNED BELOW (LINE 17). 4. Proposal prices shall include delivery of all items F.O.B. destination or as otherwise provided. 5. Address all inquiries and correspondence to the buyer at the email, phone or address shown above. 6. Email/telephone/facsimile proposals are not acceptable. 7. All instructions must be followed and signatures must be provided for proposal to be accepted. MANDATORY TO BE COMPLETED BY VENDOR 8. Payment discount terms:________________________________________ 9. Prices quoted are firm through issuance of contract until the following date________________ 10. -

CHM 220 Alkanes and Cycloalkanes
CHM 220 Alkanes and Cycloalkanes Nomenclature and Structure 1. Which of the following is not an IUPAC name for an isomer having the formula C4H9Br? (A) 1-bromobutane (B) 1-bromo-2-methylpropane (C) 2-bromobutane (D) 1-bromo-3-methylpropane 2. What is the IUPAC name for the compound having the following structure? Cl (A) 2-methyl-3-chloropentane (B) 2-methyl-3-chlorobutane (C) 1,1-dimethyl-2-chloropropane (D) 2-chloro-3-methylbutane 3. What is the IUPAC name for the compound having the following structure? (A) 2,2-diethylbutane (B) 2,2,2-triethylethane (C) 3-methyl-3-ethylpentane (D) 3-ethyl-3-methylpentane 4. Which of the following structures have the correct names? Cl Cl Cl Cl 1. tert-butyl chloride 2. isobutyl chloride 3. sec-butyl chloride 4. n-butyl chloride (A) 1 and 2 (B) 2 and 3 (C) 3 and 4 (D) 1 and 4 5. Which of the following structures have the correct names? (H3C)3COH OH OH OH 1. tert-butyl alcohol 2. sec-butyl alcohol 3. isobutyl alcohol 4. butane-3-ol (A) 1 and 2 (B) 1 and 3 (C) 2 and 4 (D) 3 and 4 6. What is the correct IUPAC name for the following structure? H3C CH3 (A) 1,2-dimethylcyclobutane (B) 1,3-dimethylcyclobutane (C) 1,4-dimethylcyclobutane (D)1,3-dimethylbicyclo[1.1.0]butane 7. What is the correct IUPAC name for the following structure? (A) bicyclo[2.1.0]hexane (B) bicyclo[2.2.1]hexane (C) bicyclo[2.2.0]hexane (D) bicyclo[3.1.0]hexane 8. -
Properties of Fuels
Appendix 1 Properties of Fuels S. McAllister et al., Fundamentals of Combustion Processes, 243 Mechanical Engineering Series, DOI 10.1007/978-1-4419-7943-8, # Springer Science+Business Media, LLC 2011 244 a b ^0 M cpg HHV LHV hfg Dh c d e Formula Fuel (kg/kmol) Tb ( C) (kJ/kg-K) Tig ( C) (MJ/kg) (MJ/kg) (kJ/kg) AFRs Tf (K) (kJ/mol) RON MON CH4 Methane 16.04 À161 2.21 537 55.536 50.048 510 17.2 2,226 À74.4 120 120 C2H2 Acetylene 26.04 À84 1.60 305 49.923 48.225 – 13.2 2,540 8.7 50 50 C2H4 Ethylene 28.05 À104 1.54 490 50.312 47.132 – 14.7 2,380 52.4 – – C2H6 Ethane 30.07 À89 1.75 472 51.902 47.611 489 16.1 2,370 À83.8 115 99 C3H8 Propane 44.10 À42 1.62 470 50.322 46.330 432 15.7 2,334 À104.7 112 97 C4H10 n-Butane 58.12 À0.5 1.64 365 49.511 45.725 386 15.5 2,270 À146.6 94 90 C4H10 iso-Butane 58.12 À12 1.62 460 49.363 45.577 366 15.5 2,310 À153.5 102 98 C5H12 n-Pentane 72.15 36 1.62 284 49.003 45.343 357 15.3 2,270 À173.5 62 63 C5H12 iso-Pentane 72.15 28 1.60 420 48.909 45.249 342 15.3 2,310 À178.5 93 90 C6H14 n-Hexane 86.18 69 1.62 233 48.674 45.099 335 15.2 2,271 À198.7 25 26 C6H14 iso-Hexane 86.18 50 1.58 421 48.454 44.879 305 15.2 2,300 À207.4 104 94 C7H16 n-Heptane 100.20 99 1.61 215 48.438 44.925 317 15.2 2,273 À224.2 0 0 C8H18 n-Octane 114.23 126 1.61 206 48.254 44.786 301 15.1 2,275 À250.1 20 17 C8H18 iso-Octane 114.23 114 1.59 418 48.119 44.651 283 15.1 2,300 À259.2 100 100 C9H20 n-Nonane 128.6 151 1.61 – 48.119 44.688 295 15.1 2,274 À274.7 – – C10H22 n-Decane 142.28 174 1.61 210 48.002 44.599 277 15.1 2,278 À300.9 À41 À38 C10H22 -
Guide to Chemical Hazard Information
GUIDE TO CHEMICAL HAZARD INFORMATION 3 1 0 EHS Ratings 2001 [v1] This Guide is intended to provide chemical hazard ratings for use with the EHS HMIS Labeling System. If a chemical is not listed, then please refer to the procedures for rating chemical hazards detailed in the EHS Chemical Hazard Reference Information Sheet and EHS HMIS Instruction Sheet. For more information contact Environmental Health and Safety at 882-7018 or visit our web site at http://web.missouri.edu/~muehs/. The information contained in this document was based on information from the Office of Radiation, Chemical & Biological Safety, Michigan State University. EHS Ratings 2001 [v1] Index Click within the blue outline to jump to the first page of chemical names that begin with the letter indicated. A M B N-O C P-R D S-T E-G U-Z H-L EHS Ratings 2001 [v1] CHEMICAL HAZARD RATINGS -- (A) Compound H F R SP HAZ Acetal 2 3 0 Acetaldehyde 3 4 2 Acetanilide 3 1 0 Acetic Acid (glacial) 3 2 0 Acetic Anhydride 3 2 1 W Acetoacetanilide 2 1 0 Acetoacet-ortho-toluidide 2 1 1 Acetoacet-para-phenetide 2 1 1 Acetone 1 3 0 Acetone cyanohydrin 4 2 2 Acetonitrile 2 3 0 Acetonyl acetone 1 1 0 Acetophenone 1 2 0 Acetyl Chloride 3 3 2 W Acetylene 0 4 3 Acetyl Peroxide. 1 2 4 Acrolein 4 3 3 Acrolein Dimer 1 2 1 Acrylamide 2 2 2 Acrylic Acid (glacial) 3 2 2 Acrylonitrile 4 3 2 Adipic Acid - 1 0 Adiponitrile 2 2 1 Adipoyl chloride 2 2 0 Adipyldinitrile 4 2 - Aldol 3 2 2 Allyl Acetate 1 3 0 Allyl Alcohol 4 3 1 Allylamine 4 3 1 Allyl Bromide 3 3 1 Allyl caproate 1 2 0 Allyl Chloride 3 3 1 Allyl -

Hazard Communication Program: Chemical Safety Manual
Hazard Communication Program: Chemical Safety Manual OSHA 1910.1200 This manual serves as the Chemical Hazard Program for Messiah University’s Grantham campus, Bowmansdale facility, and Winding Hill facility. Annual Review September 2021; Last Updated September 2021 Written Hazard Communication Program Page Messiah University strives to meet accepted guidelines and standards for accessibility and usability. If you require any information from this document in an alternate format, or are having any issues accessing the content, please contact the Office of Human Resources & Compliance at [email protected]. Table of Contents Section 1, INTRODUCTION .......................................................................................................................... 1 Section 2, DEFINITIONS ............................................................................................................................... 3 Section 3, LABELS AND OTHER FORMS OF WARNING ................................................................................. 7 Globally Harmonized System (GHS) Label ............................................................................................... 7 Pictograms .......................................................................................................................................... 8 Secondary Labels .................................................................................................................................... 8 Exceptions to Container Labeling:........................................................................................................ -

Related Properties from Molecular Structures
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2021 1 SUPPORTING INFORMATION 2 A multi-task deep learning neural network for predicting flammability- 3 related properties from molecular structures 4 Ao Yang‡a,b, Yang Su‡c,a, Zihao Wanga, Saimeng Jina, Jingzheng Renb, Xiangping Zhangd, 5 Weifeng Shen*a and James H. Clarke 6 aSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China 7 bDepartment of Industrial and Systems Engineering, The Hong Kong Polytechnic University, Hong 8 Kong SAR, China 9 cSchool of Intelligent Technology and Engineering, Chongqing University of Science and Technology, 10 Chongqing 401331, China 11 dBeijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and 12 Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China 13 eGreen Chemistry Centre of Excellence, University of York, York YO105D, UK 14 *Correspondence should be addressed to: Weifeng Shen ([email protected]) 15 ‡These authors contributed equally to this work. 16 S1. Signature descriptors 17 The signature descriptor1 is an effective tool for encoding molecular structures with all 18 atomic neighborhood into strings. This descriptor involving two forms: the first one is the 19 atomic signatures generated at a specified root atom in a molecule, the other one is the molecular 20 signatures combined from all atom signatures linearly. The size of each atomic signature is 21 determined by a parameter named “height”, h. An atomic Signature is a tree subgraph including 22 all atoms/bonds extending out to the predefined distance h without backtracking. -
Table 1: Chemicals of Concern and Associated Chemical Information
Table 1: Chemicals of Concern and Associated Chemical Information. PACs Rev. 29a, June 2018 Table 1 is an alphabetical list of the chemical substances, their Chemical Abstracts Service Registry Numbers (CASRNs)1, and pertinent physical constants. Future reviews will result in continuous updates to these data. There are also columns that provide the date of the original derivation of the PAC values, the date of the last technical review of the data used for deriving the PAC values, and the date of the last revision of the data used to derive the PAC values. The information presented in this table is derived from various sources including: . The Handbook of Chemistry and Physics Lide, D. R. (Ed.) (2009). CRC Handbook of Chemistry and Physics, CD Rom (90th Edition-2010 Version). Boca Raton, FL: Taylor and Francis. Hazardous Substances Data Bank (HSDB) The HSDB is “comprehensive, peer-reviewed toxicology data for about 5,000 chemicals” and it is accessible online at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. The Merck Index O’Neil, M. J. (Ed.) (2006). Encyclopedia of Chemicals, Drugs, and Biologicals (14th edition). Merck Research Laboratories. Whitehouse Station, NJ. SAX Lewis, R. J., Sr. (2012). Sax's Dangerous Properties of Industrial Materials (12th Edition). New York: John Wiley & Sons. Sometimes data on physicochemical properties vary among sources. As the physical constants presented in this table are obtained from published sources, users are advised to consult trusted references to verify the accuracy of the information. Information on PAC values and Temporary Emergency Exposure Limits (TEELs) and links to other sources of information are provided on the Subcommittee on Consequence Assessment and Protective Actions (SCAPA) webpage at http://orise.orau.gov/emi/scapa/default.htm provided in Table 1 is described below; it covers two pages. -
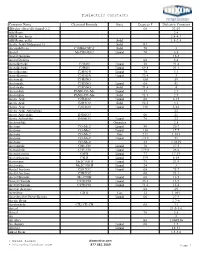
Dielectric Constants
Dielectric Constants Common Name Chemical Formula State Degrees F Dielectric Constant (Ethylenedioxy)diethanol-2,2` 68 23.69 ABS Resin 2.4 ABS Resin, lump 2.4-4.1 ABS Resin, pellet Solid 1.5-2.5 Acatic.Acid (36degrees F) Solid 4.1 Acenaphthene C10H6(CH2)2 Solid 70 3 Acetal MeCH(OEt)2 Liquid 70 3.6 Acetal Bromide 16.5 Acetal Doxime 68 3.4 Acetaldehyde C2H4O Liquid 50 21.8 Acetaldehyde C2H4O Liquid 69.8 21.1 Acetaldoxime C2H5ON Liquid 70 3.4 Acetaldoxime C2H5ON Liquid 73.4 3 Acetamide C3H5NO 180 59 Acetamide C3H5NO Liquid 68 41 Acetamide C3H5NO Solid 71.6 4 Acetanilide PhNH-CO-Me Liquid 71 2.9 Acetanilide PhNH-CO-Me Solid 71.6 2.9 Acetic Acid C2H4O2 Liquid 68 6.15 Acetic Acid C2H4O2 Solid 32.5 4.1 Acetic Acid C2H4O2 Liquid 158 6.62 Acetic Acid, Anhydrous 22 Acetic Anhydride H4H6O3 66 21 Acetic Anhydride H4H6O3 Liquid 70 22 Acetoanilide Granules 2.8 Acetone CO-Me2 Liquid 80 20.7 Acetone CO-Me2 Liquid 130 17.7 Acetone CO-Me2 Gas 212 1.015 Acetone CO-Me2 Liquid -112 31 Acetone CO-Me2 32 1.0159 Acetonitrile CH3-CN Liquid 70 37.5 Acetonitrile CH3-CN Liquid 179.6 26.6 Acetophenone C8H8 Liquid 77 17.39 Acetophenone C8H8 Liquid 394 8.64 Acetoxime Me2C:NOH Liquid 75 23.9 Acetoxime Me2C:NOH Liquid 24 3 Acetyl Acetone C5H2O2 Liquid 68 25.7 Acetyl Acetone C5H2O2 68 23.1 Acetyl Bromide Me-COBr Liquid 68 16.5 Acetyl Chloride C2H3ClO Liquid 35.6 16.9 Acetyl Chloride C2H3ClO Liquid 71.6 15.8 Acetyle Acetone 68 25 Acetylene C2H2 Gas 32 1.001 Acetylmethyl Hexyl Ketone Liquid 66 27.9 Acrylic Resin 2.7-6 Acrylonitrile CH2:CH-CN 68 33 Acteal 21.0-3.6 Acteal