The Synthesis Op Soiæe Mono-Alkyxcyclo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office Patented June 17, 1969
3,450,782 United States Patent Office Patented June 17, 1969 2 1,3-dibromocyclobutane with sodium in refluxing dioxane, 3,450,782 PROCESS FOR THE PREPARATION OF K. B. Wiberg, G. M. Lampman, R. P. Ciula, D. S. Con CYCLICALKANES nor, P. Scherter, and J. Lavanesh, Tetrahedron, 21, 2749 Daniel S. Connor, Cincinnati, Ohio, assignor to The (1695), bicyclo[1.1.1 pentane by treatment of 3-(bromo Procter & Gamble Company, Cincinnati, Ohio, a cor methyl)-cyclobutyl bromide with sodium metal at a 0.5% poration of Ohio yield, with lithium amalgam in refluxing dioxane at a No Drawing. Filed Nov. 29, 1967, Ser. No. 686,738 4.2% yield and with sodium/naphtahalene at a 8% yield, Int, C. C07c I/28 K. B. Wiberg, D. S. Connor, and G. M. Lampman, Tetra U.S. C. 260-666 8 Claims hedron Letters, 531 (1964); K. B. Wiberg and D. S. Con 0 nor, J. Am. Chem. Soc., 88, 4437 (1966). On examination of the literature hereinbefore cited ABSTRACT OF THE DISCLOSURE on cyclization, it is apparent that the yields obtained were This invention concerns the preparation of cyclic al extremely low, that the conditions for reaction when it kanes from dihalogenated alkanes using lithium amalgam. did occur were quite severe, and that the starting materials 5 used were less than common. OBJECTS OF THE INVENTION SUMMARY OF THE INVENTION The use of the method of this invention to obtain cyclic The object of this invention is to prepare cyclic al compounds provides a valuable synthetic tool heretofore kanes from the halogenated straight chain starting ma 20 unknown to chemists, thus enabling the production of terials using lithium amalgam to remove the halogens valuable end products. -

Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules
Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Cite as: Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 Published Online: 15 October 2009 Sharon G. Lias, Joel F. Liebman, and Rhoda D. Levin ARTICLES YOU MAY BE INTERESTED IN Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update Journal of Physical and Chemical Reference Data 27, 413 (1998); https:// doi.org/10.1063/1.556018 Density-functional thermochemistry. III. The role of exact exchange The Journal of Chemical Physics 98, 5648 (1993); https://doi.org/10.1063/1.464913 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu The Journal of Chemical Physics 132, 154104 (2010); https://doi.org/10.1063/1.3382344 Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 13, 695 © 1984 American Institute of Physics for the National Institute of Standards and Technology. Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Sharon G. Lias Center for Chemical Physics, National Bureau of Standards, Gaithersburg, MD 20899 Joel F. Liebman Department of Chemistry, University of Maryland Baltimore County, Catonsville, MD 21228 and Rhoda D. Levin Center for Chemical Physics. National Bureau of Standards, Gaithersburg, MD 20899 The available data on gas phase basicities and proton affinities of molecules are compiled and evaluated. Tables giving the molecules ordered (1) according to proton affinity and (2) according to empirical formula, sorted alphabetically are provided. -
![Alkanes-1 [Compatibility Mode]](https://docslib.b-cdn.net/cover/5382/alkanes-1-compatibility-mode-665382.webp)
Alkanes-1 [Compatibility Mode]
10/17/2011 Alkanes ALKANES (a “family” of hydrocarbons) CnH2n+2 CH 4 C2H6 C3H8 C4H10 etc. C2H6 ethane H H H—C—C—H H H sp 3, bond angles = 109.5 o H H σ-bonds (sigma) C C HH HH rotation about C--C (conformations) representation: “andiron” or “sawhorse” 1 10/17/2011 H H H H H H H H H H H H “staggered” “eclipsed” torsional strain: deviation from staggered. Newman projections: H H H H H H H H H H H H y g r e n e l a 3 Kcal i t n e t o p rotation about C-C The barrier to rotation about the carbon-carbon bond in ethane is 3 Kcal/mole. The rotation is ~ “free.” C3H8 propane H H H HC C C H projection formula H H H CH3CH2CH3 partially condensed formula 2 10/17/2011 CH3 H CH3 H H H H H H H H H staggered eclipsed y g r e n e l a 3.4 Kcal i t n e t o p rotation about C-C C H butane(s) 4 10 H H C H H H H H H H HC C C C H HC C C H projection H H H H H H H CH 3 partially condensed CH3CH2CH2CH3 CH3CHCH3 stick formulas Two isomers of butane C 4H10 : CH 3CH 2CH 2CH 3 n-butane bp 0 oC mp –138 oC d 0.622 g/cc CH 3 CH 3CHCH 3 isobutane bp -12 oC mp -159 oC d 0.604 g/cc 3 10/17/2011 Conformations about C2-C3 in n-butane: CH3 H CH3 H H H H H H H H C CH3 3 anti CH3/H eclipsed CH3 H3C CH3 H3C H H H H H H H H gauche CH3/CH3 eclipsed conformations about C2-C3 in n-butane: H3CCH3 CH3 H3C 4.4-6.1 Kcal y g r e n 3.4 Kcal e l 0.8 Kcal a i t CH3 n H C e 3 t CH3 o p CH3 gauche anti rotation C5H12 pentane(s) CH3CH2CH2CH2CH3 n-pentane CH3 CH3CHCH2CH3 isopentane CH3 CH3CCH3 neopentane CH3 these are common, or trivial, names where a prefix is used to idicate the structure. -

Benchmarking and Application of Density Functional Methods In
BENCHMARKING AND APPLICATION OF DENSITY FUNCTIONAL METHODS IN COMPUTATIONAL CHEMISTRY by BRIAN N. PAPAS (Under Direction the of Henry F. Schaefer III) ABSTRACT Density Functional methods were applied to systems of chemical interest. First, the effects of integration grid quadrature choice upon energy precision were documented. This was done through application of DFT theory as implemented in five standard computational chemistry programs to a subset of the G2/97 test set of molecules. Subsequently, the neutral hydrogen-loss radicals of naphthalene, anthracene, tetracene, and pentacene and their anions where characterized using five standard DFT treatments. The global and local electron affinities were computed for the twelve radicals. The results for the 1- naphthalenyl and 2-naphthalenyl radicals were compared to experiment, and it was found that B3LYP appears to be the most reliable functional for this type of system. For the larger systems the predicted site specific adiabatic electron affinities of the radicals are 1.51 eV (1-anthracenyl), 1.46 eV (2-anthracenyl), 1.68 eV (9-anthracenyl); 1.61 eV (1-tetracenyl), 1.56 eV (2-tetracenyl), 1.82 eV (12-tetracenyl); 1.93 eV (14-pentacenyl), 2.01 eV (13-pentacenyl), 1.68 eV (1-pentacenyl), and 1.63 eV (2-pentacenyl). The global minimum for each radical does not have the same hydrogen removed as the global minimum for the analogous anion. With this in mind, the global (or most preferred site) adiabatic electron affinities are 1.37 eV (naphthalenyl), 1.64 eV (anthracenyl), 1.81 eV (tetracenyl), and 1.97 eV (pentacenyl). In later work, ten (scandium through zinc) homonuclear transition metal trimers were studied using one DFT 2 functional. -

Unit 11 Halogen Derivatives
UNIT 11 HALOGEN DERIVATIVES Structure 11.1 Introduction Objectives 11.2 Classification of Halogen Derivatives 11.3 Preparation of Halogen Derivatives Alkyl Halides Aryl Halides Alkenyl Halides 11.4 Structure and Properties of Halogen Derivatives Structure of Halogen Derivatives Physical Properties of Halogen Derivatives Spectral Properties of Halogen Derivatives Chemical Properties of Alkyl Halides Chemical Properties of Aryl and Alkenyl Halides 11.5 Organometallic Compounds 11.6 Polyhalogen Derivatives Dihalogen Derivatives Trihalogen Derivatives 11.7 Uses of Halogen Derivatives 11.8 Lab Detection 11.9 Summary 11.10 Terminal Questions 11.11 Answers 11.1 INTRODUCTION In Block 2, we have described the preparation and reactions of hydrocarbons and some heterocyclic compounds. In this unit and in the next units, we will srudy some derivatives of hydrocarbons. Replacement of one or more hydrogen atoms in a hycimcarbon by halogen atom(s) [F, CI, Br, or I:] gives the halogen derivatives. These compounds are important laboratory and industrial solvents and serve as intermediates in the synthesis of other organic compounds. Many chlorohydrocarbons have acquired importance as insecticides. Although there are not many naturally occurring halogen derivatives yet you might be familiar with one such compound, thyroxin-a thyroid hormone. In this unit, we shall take up the chemistry of the halogen derivatives in detail beginning with classification of halogen derivatives and then going over to methods of their preparation. We shall also discuss the reactivity'of halogen compounds and focus our attention specially, on some important reactions such as nucleophilic substitution (SN)and elimination (E) reactions. Finally, we shall take up uses of halogen derivatives and the methods for their detection. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

Chapter 7 - Alkenes and Alkynes I
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name of acetylene 7.2 - The (E)-(Z) System for Designating Alkene Diastereomers - To determine E or Z, look at the two groups attached to one carbon atom of the double bond and decide which has higher priority. Then, repeat this at the other carbon atom. This system is not used for cycloalkenes - If the two groups of higher priority are on the same side of the double bond, the alkene is designated Z. If the two groups of higher priority are on opposite sides of the double bond, the alkene is designated E - Hydrogenation is a syn/cis addition 7.3 - Relative Stabilities of Alkenes - The trans isomer is generally more stable than the cis isomer due to electron repulsions - The addition of hydrogen to an alkene, hydrogenation, is exothermic (heat of hydrogenation) - The greater number of attached alkyl groups, the greater the stability of an alkene 7.5 - Synthesis of Alkenes via Elimination Reactions, 7.6 - Dehydrohalogenation of Alkyl Halides How to Favor E2: - Reaction conditions that favor elimination by E1 should be avoided due to the highly competitive SN 1 mechanism - To favor E2, a secondary or tertiary alkyl halide should be used - If there is only a possibility for a primary alkyl halide, use a bulky base - Use a higher concentration of a strong and nonpolarizable base, like an alkoxide - EtONa=EtOH favors the more substituted double bond -
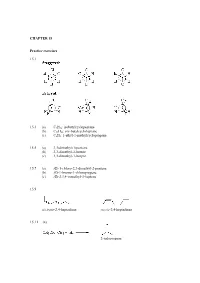
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

Organic Seminar Abstracts
Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemin1971752univ / a ORGANIC SEMINAR ABSTRACTS 1971-1972 Semester II School of Chemical Sciences Department of Chemistry- University of Illinois Urbana, Illinois 3 SEMINAR TOPICS II Semester 1971-72 Reactions of Alkyl Ethers Involving n- Complexes on a Reaction Pathway 125 Jerome T. Adams New Syntheses of Helicenes 127 Alan Morrice Recent Advances in Drug Detection and Analysis I36 Ronald J. Convers The Structure of Carbonium Ions with Multiple Neighboring Groups 138 William J. Work Recent Reactions of the DMSO-DCC Reagent ll+O James A. Franz Nucleophilic Acylation 1^2 Janet Ollinger The Chemistry of Camptothecin lU^ Dale Pigott Stereoselective Syntheses and Absolute Configuration of Cecropia Juvenile Horomone 1U6 John C. Greenfield Uleine and Related Aspidosperma Alkaloids 155 Glen Tolman Strategies in Oligonucleotide Synthesis 162 Graham Walker Stable Carbocations: C-13 Nuclear Magnetic Resonance Studies 16U Moses W. McMillan Organic Chlorosulfinates 166 Steven W. Moje Recent Advances in the Chemistry of Penicillins and Cephalosporins 168 Ronald Stroshane Cerium (iv) Oxidations 175 William C. Christopfel A New Total Synthesis of Vitamin D 18 William Graham Ketone Transpositions 190 Ann L. Crumrine - 125 - REACTIONS OF ALKYL ETHERS INVOLVING n-COMPLEXES ON A REACTION PATHWAY Reported by Jerome T. Adams February 2k 1972 The n-complex (l) has been described as an intermediate on the reaction pathway for electrophilic aromatic substitution and acid catalyzed rearrange- ment of alkyl aryl ethers along with sigma (2) and pi (3) type intermediates. 1 ' 2 +xR Physical evidence for the existence of n-complexes of alkyl aryl ethers was found in the observation of methyl phenyl ononium ions by nmr 3 and ir observation of n-complexes of anisole with phenol. -

Elimination Reactions Are Described
Introduction In this module, different types of elimination reactions are described. From a practical standpoint, elimination reactions widely used for the generation of double and triple bonds in compounds from a saturated precursor molecule. The presence of a good leaving group is a prerequisite in most elimination reactions. Traditional classification of elimination reactions, in terms of the molecularity of the reaction is employed. How the changes in the nature of the substrate as well as reaction conditions affect the mechanism of elimination are subsequently discussed. The stereochemical requirements for elimination in a given substrate and its consequence in the product stereochemistry is emphasized. ELIMINATION REACTIONS Objective and Outline beta-eliminations E1, E2 and E1cB mechanisms Stereochemical considerations of these reactions Examples of E1, E2 and E1cB reactions Alpha eliminations and generation of carbene I. Basics Elimination reactions involve the loss of fragments or groups from a molecule to generate multiple bonds. A generalized equation is shown below for 1,2-elimination wherein the X and Y from two adjacent carbon atoms are removed, elimination C C C C -XY X Y Three major types of elimination reactions are: α-elimination: two atoms or groups are removed from the same atom. It is also known as 1,1-elimination. H R R C X C + HX R Both H and X are removed from carbon atom here R Carbene β-elimination: loss of atoms or groups on adjacent atoms. It is also H H known as 1,2- elimination. R C C R R HC CH R X H γ-elimination: loss of atoms or groups from the 1st and 3rd positions as shown below. -

Maximum Topological Distances Based Indices As Molecular Descriptors for QSPR
Int. J. Mol. Sci. 2001, 2, 121-132 International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.org/ijms/ Maximum Topological Distances Based Indices as Molecular Descriptors for QSPR. 4. Modeling the Enthalpy of Formation of Hydrocarbons from Elements Andrés Mercader 1, Eduardo A. Castro 1,* and Andrey A. Toropov2 1 CEQUINOR, Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de la Plata, C.C. 962, La Plata 1900, Argentina. e-mail: [email protected] 2 Andrey A. Toropov, Vostok Innovation Company, S. Azimstreet 4, 100047 Tashkent, Uzbekistan. e-mail: [email protected] * Author to whom correspondence should be addressed. e-mail: [email protected] Received: 9 April 2001 Accepted: 15 June 2001/ Published: 28 June 2001 Abstract: The enthalpy of formation of a set of 60 hydroarbons is calculated on the basis of topological descriptors defined from the distance and detour matrices within the realm of the QSAR/QSPR theory. Linear and non-linear polynomials fittings are made and results show the need to resort to higher-order regression equations in order to get better concordances between theoretical results and experimental available data. Besides, topological indices computed from maximum order distances seems to yield rather satisfactory predictions of heats of formation for hydrocarbons. Keywords: QSPR theory, Detour matrix, Enthalpy of formation, Hydrocarbons, Topologi- cal indices 1. Introduction Graphs have found considerable employment in several chemistry fields, particularly in modeling molecular structure [1-10]. The applications of graphs to the study of structure-property relationships implies the representation of molecules by selected molecular descriptors, often referred to as topo- logical indices [11,12]. -

Organometallics in Regio Enantio-Selective Synthes
THESIS IN JOINT SUPE RVISION DOTTORATO IN SCIENZE CHIMICHE DOCTORAT DE L’UNIVERSITE DE ROUEN SETTORE DISCIPLINARE CHIMICA ORGANICA CHIM/06 Spécialité: CHIMIE XXII CICLO Discipline: CHIMIE ORGANIQUE Organometallics in Regio --- aaandand EnantioEnantio----Selective Synthesis: Structures and Reactivity Gabriella Barozzino Consiglio members of jury Prof. Giovanni POLI (Rapporteur) Université Pierre et Marie Curie (Paris VI) Prof. Paolo VENTURELLO (Rapporteur) Università di Torino Prof. Annie-Claude GAUMONT (Examin er) Université de Caen Prof. Vito CAPRIATI (Examiner) Università di Bari Prof. Hassan OULYADI (Directeur de Thèse à Rouen) Université de Rouen Dr. Alessandro MORDINI (Direttore di Tesi a Firenze) Università di Firenze 2007-2009 INDEX Abbreviations and Acronyms vii Abstracts 1 1. General Introduction 3 1.1. Introduction 3 1.2. Aim of the project 5 1.3. Organolithium chemistry 7 1.3.1. Organolithium structures 7 1.3.2. Organolithium aggregates 8 1.3.3. Dynamic of organolithium compounds 10 1.3.4. Analysis of organolithium compounds 12 First Part 2. Introduction to the Base-Promoted Rearrangement of Strained Heterocycles 15 2.1. Superbases 15 2.1.1. Structures and reactivity 16 2.1.2. Applications 18 2.2. Epoxides and aziridines 20 2.2.1. Epoxides 21 2.2.2. Aziridines 22 2.3. Previous results about the base-induced isomerizations of epoxides 23 2.4. Previous results about the base-induced isomerizations of epoxy ethers 26 2.5. Previous results about the base-induced isomerizations of aziridines 30 2.5.1. Isomerizations of aziridines 31 2.5.2. Elaboration of allylic amines 34 i Index 3. Rearrangements of Aziridinyl Ethers Mediated by Superbases 37 3.1.