Trend Analysis for Atmospheric Hydrocarbon Partitioning Using Continuous Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office Patented June 17, 1969
3,450,782 United States Patent Office Patented June 17, 1969 2 1,3-dibromocyclobutane with sodium in refluxing dioxane, 3,450,782 PROCESS FOR THE PREPARATION OF K. B. Wiberg, G. M. Lampman, R. P. Ciula, D. S. Con CYCLICALKANES nor, P. Scherter, and J. Lavanesh, Tetrahedron, 21, 2749 Daniel S. Connor, Cincinnati, Ohio, assignor to The (1695), bicyclo[1.1.1 pentane by treatment of 3-(bromo Procter & Gamble Company, Cincinnati, Ohio, a cor methyl)-cyclobutyl bromide with sodium metal at a 0.5% poration of Ohio yield, with lithium amalgam in refluxing dioxane at a No Drawing. Filed Nov. 29, 1967, Ser. No. 686,738 4.2% yield and with sodium/naphtahalene at a 8% yield, Int, C. C07c I/28 K. B. Wiberg, D. S. Connor, and G. M. Lampman, Tetra U.S. C. 260-666 8 Claims hedron Letters, 531 (1964); K. B. Wiberg and D. S. Con 0 nor, J. Am. Chem. Soc., 88, 4437 (1966). On examination of the literature hereinbefore cited ABSTRACT OF THE DISCLOSURE on cyclization, it is apparent that the yields obtained were This invention concerns the preparation of cyclic al extremely low, that the conditions for reaction when it kanes from dihalogenated alkanes using lithium amalgam. did occur were quite severe, and that the starting materials 5 used were less than common. OBJECTS OF THE INVENTION SUMMARY OF THE INVENTION The use of the method of this invention to obtain cyclic The object of this invention is to prepare cyclic al compounds provides a valuable synthetic tool heretofore kanes from the halogenated straight chain starting ma 20 unknown to chemists, thus enabling the production of terials using lithium amalgam to remove the halogens valuable end products. -

Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules
Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Cite as: Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 Published Online: 15 October 2009 Sharon G. Lias, Joel F. Liebman, and Rhoda D. Levin ARTICLES YOU MAY BE INTERESTED IN Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update Journal of Physical and Chemical Reference Data 27, 413 (1998); https:// doi.org/10.1063/1.556018 Density-functional thermochemistry. III. The role of exact exchange The Journal of Chemical Physics 98, 5648 (1993); https://doi.org/10.1063/1.464913 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu The Journal of Chemical Physics 132, 154104 (2010); https://doi.org/10.1063/1.3382344 Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 13, 695 © 1984 American Institute of Physics for the National Institute of Standards and Technology. Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Sharon G. Lias Center for Chemical Physics, National Bureau of Standards, Gaithersburg, MD 20899 Joel F. Liebman Department of Chemistry, University of Maryland Baltimore County, Catonsville, MD 21228 and Rhoda D. Levin Center for Chemical Physics. National Bureau of Standards, Gaithersburg, MD 20899 The available data on gas phase basicities and proton affinities of molecules are compiled and evaluated. Tables giving the molecules ordered (1) according to proton affinity and (2) according to empirical formula, sorted alphabetically are provided. -

Benchmarking and Application of Density Functional Methods In
BENCHMARKING AND APPLICATION OF DENSITY FUNCTIONAL METHODS IN COMPUTATIONAL CHEMISTRY by BRIAN N. PAPAS (Under Direction the of Henry F. Schaefer III) ABSTRACT Density Functional methods were applied to systems of chemical interest. First, the effects of integration grid quadrature choice upon energy precision were documented. This was done through application of DFT theory as implemented in five standard computational chemistry programs to a subset of the G2/97 test set of molecules. Subsequently, the neutral hydrogen-loss radicals of naphthalene, anthracene, tetracene, and pentacene and their anions where characterized using five standard DFT treatments. The global and local electron affinities were computed for the twelve radicals. The results for the 1- naphthalenyl and 2-naphthalenyl radicals were compared to experiment, and it was found that B3LYP appears to be the most reliable functional for this type of system. For the larger systems the predicted site specific adiabatic electron affinities of the radicals are 1.51 eV (1-anthracenyl), 1.46 eV (2-anthracenyl), 1.68 eV (9-anthracenyl); 1.61 eV (1-tetracenyl), 1.56 eV (2-tetracenyl), 1.82 eV (12-tetracenyl); 1.93 eV (14-pentacenyl), 2.01 eV (13-pentacenyl), 1.68 eV (1-pentacenyl), and 1.63 eV (2-pentacenyl). The global minimum for each radical does not have the same hydrogen removed as the global minimum for the analogous anion. With this in mind, the global (or most preferred site) adiabatic electron affinities are 1.37 eV (naphthalenyl), 1.64 eV (anthracenyl), 1.81 eV (tetracenyl), and 1.97 eV (pentacenyl). In later work, ten (scandium through zinc) homonuclear transition metal trimers were studied using one DFT 2 functional. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -
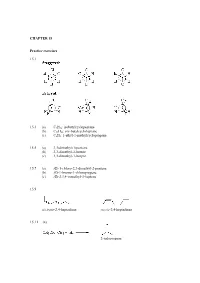
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

Maximum Topological Distances Based Indices As Molecular Descriptors for QSPR
Int. J. Mol. Sci. 2001, 2, 121-132 International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.org/ijms/ Maximum Topological Distances Based Indices as Molecular Descriptors for QSPR. 4. Modeling the Enthalpy of Formation of Hydrocarbons from Elements Andrés Mercader 1, Eduardo A. Castro 1,* and Andrey A. Toropov2 1 CEQUINOR, Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de la Plata, C.C. 962, La Plata 1900, Argentina. e-mail: [email protected] 2 Andrey A. Toropov, Vostok Innovation Company, S. Azimstreet 4, 100047 Tashkent, Uzbekistan. e-mail: [email protected] * Author to whom correspondence should be addressed. e-mail: [email protected] Received: 9 April 2001 Accepted: 15 June 2001/ Published: 28 June 2001 Abstract: The enthalpy of formation of a set of 60 hydroarbons is calculated on the basis of topological descriptors defined from the distance and detour matrices within the realm of the QSAR/QSPR theory. Linear and non-linear polynomials fittings are made and results show the need to resort to higher-order regression equations in order to get better concordances between theoretical results and experimental available data. Besides, topological indices computed from maximum order distances seems to yield rather satisfactory predictions of heats of formation for hydrocarbons. Keywords: QSPR theory, Detour matrix, Enthalpy of formation, Hydrocarbons, Topologi- cal indices 1. Introduction Graphs have found considerable employment in several chemistry fields, particularly in modeling molecular structure [1-10]. The applications of graphs to the study of structure-property relationships implies the representation of molecules by selected molecular descriptors, often referred to as topo- logical indices [11,12]. -

Cometabolic Degradation of Polycyclic Aromatic Hydrocarbons (Pahs) and Aromatic Ethers by Phenol- and Ammonia-Oxidizing Bacteria
AN ABSTRACT OF THE DISSERTATION OF Soon Woong Chang for the degree of Doctor of Philosophy in Civil Engineering presented on November 11, 1997. Title: Cometabolic Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) and Aromatic Ethers by Phenol- and Ammonia-Oxidizing Bacteria Redacted for Privacy Abstract approved: /Kenneth J. Williamson Cometabolic biodegradation processes are potentially useful for the bioremediation of hazardous waste sites. In this study the potential application of phenol- oxidizing and nitrifying bacteria as "priming biocatalysts" was examined in the degradation of polycyclic aromatic hydrocarbons (PAHs), aryl ethers, and aromatic ethers. We observed that a phenol-oxidizing Pseudomonas strain cometabolically degrades a range of 2- and 3-ringed PAHs. A sequencing batch reactor (SBR) was used to overcome the competitive effects between two substrates and the SBR was evaluated as a alternative technology to treat mixed contaminants including phenol and PAHs. We also have demonstrated that the nitrifying bacterium Nitrosomonas europaea can cometabolically degrade a wide range polycyclic aromatic hydrocarbons (PAHs), aryl ethers and aromatic ethers including naphthalene, acenaphthene, diphenyl ether, dibenzofuran, dibenzo-p-dioxin, and anisole. Our results indicated that all the compounds are transformed by N. europaea and that several unusual reactions are involved in these reactions. In the case of naphthalene oxidation, N. europaea generated predominantly 2 naphthol whereas other monooxygenases generate 1-naphthol as the major product. In the case of dibenzofuran oxidation, 3-hydroxydibenzofuran initially accumulated in the reaction medium and was then further transformed to 3-hydroxy nitrodibenzofuran in a pH- and nitrite-dependent abiotic reaction. A similar abiotic transformation reaction also was observed with other hydroxylated aryl ethers and PAHs. -

Incomplete Draft Documentation of The
INCOMPLETE DRAFT DOCUMENTATION OF THE SAPRC-16 MECHANISM GENERATION SYSTEM Interim Report to California Air Resources Board Contract No. 11-761 William P. L. Carter May 25, 2019 College of Engineering Center for Environmental Research and Technology (CE-CERT) University of California, Riverside, California 92521 SUMMARY This document gives a description of the automated chemical mechanism generation system that is used to derive gas-phase atmospheric reaction mechanisms for reactive organic compounds for use in atmospheric chemical models. In particular, this system is used to derive the SAPRC-16 that is being developed. The system, called MechGen uses a computer program to derive fully explicit mechanisms for the reactions of many types of emitted organic compounds and their oxidation products when they react in the atmosphere in the presence of oxides of nitrogen and other pollutants, and then uses the results to derive lumped mechanisms suitable for use in atmospheric models. This report gives an overview of system, describes the procedures used to generate detailed explicit mechanisms, and the procedures used to derive lumped reactions for the SAPRC-16 chemical mechanism for atmospheric modeling. The scientific basis for the generation of the detailed mechanisms, including the assumptions, approximations, and assignments or estimations of the rate constants or branching ratios involved, are described in a separate document (Carter, 2019a). The system runs on a programming platform originally developed for text-based and is accessible by anyone over the internet using a web-based interface, with a telnet login being used to program and manage the system and input assignments. The web-based interface can be used to generate complete mechanisms for selected compounds or to examine individual reactions to see documentation output concerning how individual reactions and rate constants are derived. -

Cyclopropanation Chem 115
Myers Cyclopropanation Chem 115 Reviews: • Bonding Orbitals in Cyclopropane (Walsh Model): Roy, M.-N.; Lindsay, V. N. G.; Charette, A. B. Stereoselective Synthesis: Reactions of Carbon– Carbon Double Bonds (Science of Synthesis); de Vries, J. G., Ed.; Thieme: Stuttgart, 2011, Vol 1.; 731–817. Lebel, H.; Marcoux, J.-F.; Molinaro, C.; Charette, A. B. Chem. Rev. 2003, 103, 977–1050. Davies, H. M. L.; Beckwith, R. E. J. Chem. Rev. 2003, 103, 2861–2903. Li, A-H.; Dai, L. X.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2341–2372. eS (") eA (") • Applications of Cyclopropanes in Synthesis Carson, C. A.; Kerr, M. A. Chem. Soc. Rev. 2009, 38, 3051–3060. Reissig, H.-U.; Zimmer, R. Chem. Rev. 2003, 103, 1151–1196. Gnad, F.; Reiser, O. Chem. Rev. 2003, 103, 1603–1624. • Cyclopropane Biosynthesis ! Thibodeaux, C. J.; Chang, W.-c.; Liu, H.-w. Chem. Rev. 2012, 112, 1681–1709. de Meijere, A. Angew. Chem. Int. Ed. 1979, 18, 809–886. General Strategies for Cyclopropanation: Introduction • via carbenoids "MCH2X" H HH H H H • via carbenes generated by decomposition of diazo compounds H H H H H H RCHN2 • Cyclopropanes are stable but highly strained compounds (ring strain ~29 kcal/mol). R • via Michael addition and ring closure • C–C bond angles = 60º (vs 109.5º for normal Csp3–Csp3 bonds). • Substituents on cyclopropanes are eclipsed. H–C–H angle is ~120º. As a result, the C–H bonds RCH–LG have higher s character compared to normal sp3 bonds. EWG EWG EWG LG R • Because of their inherent strain, the reactivity of cyclopropanes is more closely analogous to that of alkenes than that of alkanes. -

An Analysis of the Vibrations of Certain Large Molecules
AN ANALYSIS OF THE VIBRATIONS OF CERTAIN LARGE MOLECULES INCLUDING SPIROPENTANE, METHYLENECYCLOPROPANE} AND CYCLOPROPANE DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By WALTER BERNARD LOEWENSTEIN, B.S. The Ohio State University 195^ Approved by: Adviser ACKNOWLEDGMENT The author wishes to express his sincere grat itude to Professor W. H. Shaffer for his guidance, counsel and encouragement throughout the recent years. Pie would also like to express his thanks to the Texas Company for the award of a fellowship during the tenure of which this work was completed He is also grateful for the constant help and en couragement given by his parents. i TABLE OF CONTENTS >ag I INTRODUCTION 1 II THE SPIROPENTANE MOLECULE 5 Introduction 5 The Multiple Origin Method 5 The Structure of Spiropentane 7 Coordinates 9 III SYMMETRY PROPERTIES OF SPIROPENTANE 16 Group Theoretical Predictions 16 Covering Operations 19 Symmetry Coordinates 21 IV THE SECULAR DETERMINANT OF SPIROPENTANE 31 Introduction 31 The Kinetic Energy 31 The Potential Energy 3b The Secular Determinant k-5 V FREQUENCY ASSIGNMENTS AND CALCULATIONS 53 Possible Assignments 53 Calculations 59 More Definite Assignments 66 VI THE METHYLENECYC LOPROPANE MOLECULE 69 Introduction 69 The Geometry 69 Coordinate Systems Ik Symmetry Coordinates 78 Eckart Conditions 8 0 iii TABLE OF CONTENTS (Continued) Page CHAPTER VII THE SECULAR DETERMINANT OF METHYLENE- CYCLOPROPANE 85 A. Introduction 85 B. The Kinetic Energy 86 C. The Potential Energy 91 D. The Secular Determinant 95 CHAPTER VIII FREQUENCY ASSIGNMENTS AND CALCULATIONS 98 A. -

Synthesis and Reactivity of Small Ring Bicyclic Compounds Raymond Lee Welch Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1969 Synthesis and reactivity of small ring bicyclic compounds Raymond Lee Welch Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Welch, Raymond Lee, "Synthesis and reactivity of small ring bicyclic compounds " (1969). Retrospective Theses and Dissertations. 3807. https://lib.dr.iastate.edu/rtd/3807 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 70-7767 WELCH, Raymond Lee, 1948- SYNTHESIS AND REACTIVITY OF SMALL RING BICYCLIC COMPOUNDS. Iowa State University, PhJD., 1969 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan SYNTHESIS AND REACTIVITY OF SMALL RING BICYCLIC COMPOUNDS by Raymond Lee Welch A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved : Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Signature was redacted for privacy. Iowa State University Of Science and Technology -

Chapter 21 Organic Chemistry
CHAPTER 21 ORGANIC CHEMISTRY Hydrocarbons 1. A hydrocarbon is a compound composed of only carbon and hydrogen. A saturated hydro- carbon has only carbon-carbon single bonds in the molecule. An unsaturated hydrocarbon has one or more carbon-carbon multiple bonds but may also contain carbon-carbon single bonds. A normal hydrocarbon has one chain of consecutively bonded carbon atoms. A branched hydrocarbon has at least one carbon atom not bonded to the end carbon of a chain of consecutively bonded carbon atoms. Instead, at least one carbon atom forms a bond to an inner carbon atom in the chain of consecutively bonded carbon atoms. 2. To determine the number of hydrogens bonded to the carbons in cyclic alkanes (or any alkane where they may have been omitted), just remember that each carbon has four bonds. In cycloalkanes, only the C−C bonds are shown. It is assumed you know that the remaining bonds on each carbon are C−H bonds. The number of C−H bonds is that number required to give the carbon four total bonds. 3. In order to form, cyclopropane and cyclobutane are forced to form bond angles much smaller than the preferred 109.5° bond angles. Cyclopropane and cyclobutane easily react in order to obtain the preferred 109.5° bond angles. 4. Aromatic hydrocarbons are a special class of unsaturated hydrocarbons based on the benzene ring. Benzene has the formula C6H6. It is a planar molecule (all atoms are in the same plane). Each carbon in benzene is attached to three other atoms; it exhibits trigonal planar geometry with 120° bond angles.