Cometabolic Degradation of Polycyclic Aromatic Hydrocarbons (Pahs) and Aromatic Ethers by Phenol- and Ammonia-Oxidizing Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Biofuel Policies in Select Eu Member States: 2018 Update
© INTERNATIONAL COUNCIL ON CLEAN TRANSPORTATION POLICY UPDATE NOVEMBER 2018 ADVANCED BIOFUEL POLICIES IN SELECT EU MEMBER STATES: 2018 UPDATE This policy update provides details on the latest measures that select European ICCT POLICY UPDATES Union (EU) member states, namely Denmark, Germany, Italy, the Netherlands, SUMMARIZE Sweden, and the United Kingdom, are taking to support advanced alternative fuels. REGULATORY AND OTHER EU POLICY BACKGROUND DEVELOPMENTS In 2018, the European Union (EU) set its climate and energy objectives for 2030. RELATED TO CLEAN They included a greenhouse gas (GHG) reduction of at least 40% and a minimum of a TRANSPORTATION 32% share of renewable energy consumption across all sectors.1 GHG emissions in the WORLDWIDE. European transportation sector have declined by only 3.8% since 2008, compared to an 18% decrease, or more, in all other sectors, indicating that the decarbonization of transportation should be a priority for the future.2 Biofuels are one of the options considered to increase renewable energy and decrease the carbon intensity of the transportation sector. Through the use of directives and national legislation, the EU has incentivized both the adoption of conventional food-based biofuels and advanced biofuels, which are made from non-food feedstocks. Such incentives date to 2009, when the EU Renewable Energy Directive (RED) mandated that by 2020, 10% of energy used in the transportation sector should come from renewable energy sources (RES).3 In 2015, the RED was 1 Jacopo Giuntoli, Final recast Renewable Energy Directive for 2021-2030 in the European Union, (ICCT: Washington, DC, 2018), https://www.theicct.org/publications/final-recast-renewable-energy-directive- 2021-2030-european-union 2 EUROSTAT (Greenhouse gas emissions by source sector (env_air_gge), accessed November 2018), https://ec.europa.eu/eurostat. -

SYNTHESIS and PROPERTIES of SOME ARALKYL Hymoperoxides
SYNTHESIS AND PROPERTIES OF SOME ARALKYL HYmOPEROXIDES DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By ARLO d / bCGGS, B.S., M.S. The Ohio State University 1954 Approved by: Department of Chemistry ACKNOWLEDGEMENT The author wishes to express sincere appreciation to Professor Cecil E. Boord for his advice and counsel during this investigation* Thanks also are due Dr. Kenneth W*. Greenlee for his continual interest and guidance and his cooperation in ex tending the facilities of the American Petroleum Institute Research Project 4-5* The financial support of this work by the Firestone Tire and Rubber Company is gratefully acknowledged* ii TABLE OF CONTENTS Page I. INTRODUCTION................................ 1 II. LITERATURE S URVEY ............... 2 III. STATEMENT OF THE PROBLEM.................... 10 IV. DISCUS5IŒ ........................... 11 A. Methods of Preparing Hydroperoxides .... 12 1. Preparation of hydroperoxides from alcohols ............. 12 a. a-methylbenzyl hydroperoxide ...... 12 b. benzyl hydroperoxide .......... l6 c. cinnamyl and a-phenylallyl hydroperoxides 17 d. 1,2,3,4-tetrahydro-l-naphthyl hydro peroxide ............ 22 e. a-indanyl hydroperoxide ........ 23 f. 0-, m- and p-methylbenzyl hydroperoxides 24 g. m- and p-methoxybenzyl hydroperoxides. 28 h. 1,1-diphenylmethyl hydroperoxide .... 31 i. 1,2-diphenylethyl hydroperoxide .... 32 j. 1-a-naphthyl- and l-J3-naphthylethyl hydroperoxides ........ 33 k. 1-styrylethyl hydroperoxide 35 1. 4-a-dimethylbenzyl and 4-methoxy-a- methylbenzyl hydroperoxides ...... 36 m. a-ethylbenzyl and a-ethyl-p-methylbenzyl hydroperoxides ....... ........ 36 n. a-n-propylbenzyl and a-isopropylbenzyl hydroperoxides .................... 37 0. a-2,5“trimethylbenzyl hydroperoxide . -

Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules
Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Cite as: Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 Published Online: 15 October 2009 Sharon G. Lias, Joel F. Liebman, and Rhoda D. Levin ARTICLES YOU MAY BE INTERESTED IN Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update Journal of Physical and Chemical Reference Data 27, 413 (1998); https:// doi.org/10.1063/1.556018 Density-functional thermochemistry. III. The role of exact exchange The Journal of Chemical Physics 98, 5648 (1993); https://doi.org/10.1063/1.464913 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu The Journal of Chemical Physics 132, 154104 (2010); https://doi.org/10.1063/1.3382344 Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 13, 695 © 1984 American Institute of Physics for the National Institute of Standards and Technology. Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Sharon G. Lias Center for Chemical Physics, National Bureau of Standards, Gaithersburg, MD 20899 Joel F. Liebman Department of Chemistry, University of Maryland Baltimore County, Catonsville, MD 21228 and Rhoda D. Levin Center for Chemical Physics. National Bureau of Standards, Gaithersburg, MD 20899 The available data on gas phase basicities and proton affinities of molecules are compiled and evaluated. Tables giving the molecules ordered (1) according to proton affinity and (2) according to empirical formula, sorted alphabetically are provided. -

One-Pot Syntheses of Irida-Polycyclic Aromatic Hydrocarbons† Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue One-pot syntheses of irida-polycyclic aromatic hydrocarbons† Cite this: Chem. Sci.,2019,10, 10894 a a a b a All publication charges for this article Yu Xuan Hu,‡ Jing Zhang,‡ Xiaoyan Wang, Zhengyu Lu, Fangfang Zhang, have been paid for by the Royal Society Xiaofei Yang,a Zhihua Ma,a Jun Yin, *a Haiping Xia b and Sheng Hua Liu *a of Chemistry Metalla-analogues of polycyclic aromatic hydrocarbons (PAHs) have captivated chemists with their fascinating structures and unique electronic properties. To date, metallabenzene, metallanaphthalene and metallaanthracene have been reported. Metalla-analogues with more complicated fused rings have rarely been reported. Herein, we have successfully synthesized a series of new iridafluoranthenes and fused-ring iridafluoranthenes ranging from pentacyclic to heptacyclic metallaaromatic hydrocarbons in Received 6th August 2019 high yields under mild reaction conditions for the first time. Their photophysical and redox properties Accepted 12th October 2019 were also explored using UV-vis spectroscopy and electrochemistry combined with TD-DFT DOI: 10.1039/c9sc03914g calculations. The present work may offer an important guideline for the design and construction of new rsc.li/chemical-science polycyclic metallaaromatic hydrocarbons and metalla-nanographenes. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Introduction carbeneiridium compound by using an intramolecular C–H activation reaction.6b In 2018, they further developed irida- Polycyclic aromatic hydrocarbons (PAHs), as important compo- phenanthrene, iridanaphthalene and iridaanthracene from nents in the eld of organic chemistry, have attracted a signi- their corresponding methoxy(alkenyl)carbeneiridium inter- * cant amount of attention due to their wide range of applications mediates via reactions of [IrCp Cl(NCMe) (PMe3)]PF6 with 8 in organic light-emitting diodes,1 eld-effect transistors2 and diarylpropargyl alcohols. -

Bringing Biofuels on the Market
Bringing biofuels on the market Options to increase EU biofuels volumes beyond the current blending limits Report Delft, July 2013 Author(s): Bettina Kampman (CE Delft) Ruud Verbeek (TNO) Anouk van Grinsven (CE Delft) Pim van Mensch (TNO) Harry Croezen (CE Delft) Artur Patuleia (TNO) Publication Data Bibliographical data: Bettina Kampman (CE Delft), Ruud Verbeek (TNO), Anouk van Grinsven (CE Delft), Pim van Mensch (TNO), Harry Croezen (CE Delft), Artur Patuleia (TNO) Bringing biofuels on the market Options to increase EU biofuels volumes beyond the current blending limits Delft, CE Delft, July 2013 Fuels / Renewable / Blends / Increase / Market / Scenarios / Policy / Technical / Measures / Standards FT: Biofuels Publication code: 13.4567.46 CE Delft publications are available from www.cedelft.eu Commissioned by: The European Commission, DG Energy. Further information on this study can be obtained from the contact person, Bettina Kampman. Disclaimer: This study Bringing biofuels on the market. Options to increase EU biofuels volumes beyond the current blending limits was produced for the European Commission by the consortium of CE Delft and TNO. The views represented in the report are those of its authors and do not represent the views or official position of the European Commission. The European Commission does not guarantee the accuracy of the data included in this report, nor does it accept responsibility for any use made thereof. © copyright, CE Delft, Delft CE Delft Committed to the Environment CE Delft is an independent research and consultancy organisation specialised in developing structural and innovative solutions to environmental problems. CE Delft’s solutions are characterised in being politically feasible, technologically sound, economically prudent and socially equitable. -

Detection of Phenethylamine, Amphetamine, and Tryptamine Imine By-Products from an Acetone Extraction
Detection of Phenethylamine, Amphetamine, and Tryptamine Imine By-Products from an Acetone Extraction Mary A. Yohannan* and Arthur Berrier U.S. Department of Justice Drug Enforcement Administration Special Testing and Research Laboratory 22624 Dulles Summit Court Dulles, VA 20166 [email: mary.a.yohannan -at- usdoj.gov] ABSTRACT: The formation of imine by-products from phenethylamines, amphetamines, and tryptamines upon an acetone extraction is presented. These imine by-products were characterized using GC/MSD and exhibited preferential cleavage at the α-carbon of the alkyl chain. Further characterization of the imine by-products of phenethylamine and tryptamine was done using IR and NMR. KEYWORDS: phenethylamine, tryptamine, imine, acetone, schiff base, drug chemistry, forensic chemistry In most forensic laboratories, the solvents used to extract at the α-carbon on the alkyl chain. In addition to GC/MS, the drugs are chosen based upon their solubility properties and their imines formed from phenethylamine base and tryptamine base ability to not interact with the drug. In fact, there are very few were characterized by Fourier transform-infrared spectroscopy publications where a solvent used to extract a drug reacts with (FTIR) and nuclear magnetic resonance (NMR) spectroscopy. the drug and forms by-products [1-3]. This laboratory recently discovered that an additional Experimental component was formed when acetone was used to extract a Solvents, Chemicals, and Materials sample containing a known tryptamine. Analysis by gas Acetone was ACS/HPLC grade from Burdick and Jackson chromatography/mass spectroscopy (GC/MS) of the acetone Laboratories (Muskegon, MI). Phenethylamine base and extract yielded an extra peak in the total ion chromatogram that tryptamine base were obtained from Sigma-Aldrich Chemicals was approximately half the abundance of the known tryptamine (Milwaukee, WI). -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

Gas Phase Ion/Molecule Reactions As Studied by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry
INIS-mf—10165 GAS PHASE ION/MOLECULE REACTIONS AS STUDIED BY FOURIER TRANSFORM ION CYCLOTRON RESONANCE MASS SPECTROMETRY STEEN INGEMANN J0RGENSEN GAS PHASE ION/MOLECULE REACTIONS AS STUDIED BY FOURIER TRANSFORM ION CYCLOTRON RESONANCE MASS SPECTROMETRY ACADEMISCH PROEFSCHRIFT ter verkrijging van de graad van doctor in de Wiskunde en Natuurwetenschappen aan de Universiteit van Amsterdam, op gezag van de Rector Magnificus dr. D.W. Bresters, hoogleraar in de Faculteit der Wiskunde en Natuurwetenschappen, in het openbaar te verdedigen in de Aula der Universiteit (tijdelijk in het Wiskundegebouw, Roetersstraat 15) op woensdag 12 juni 1985 te 16.00 uur. door STEEN INGEMANN J0RGENSEN geboren te Kopenhagen 1985 Offsetdrukkerij Kanters B.V., Alblasserdam PROMOTOR: Prof. Dr. N.M.M. Nibbering Part of the work described in this thesis has been accomplished under the auspices of the Netherlands Foundation for Chemical Research (SON) with the financial support from the Netherlands Organization for the Advancement of Pure Research (ZWO). STELLINGEN 1. De door White e.a. getrokken conclusie, dat het cycloheptatrieen anion omlegt tot het benzyl anion in de gasfase, is onvoldoende ondersteund door de experimentele gegevens. R.L. White, CL. Wilkins, J.J. Heitkamp, S.W. Staley, J. Am. Chem. Soc, _105, 4868 (1983). 2. De gegeven experimentele methode voor de lithiëring van endo- en exo- -5-norborneen-2,3-dicarboximide is niet in overeenstemming met de ge- postuleerde vorming van een algemeen dianion van deze verbindingen. P.J. Garratt, F. Hollowood, J. Org. Chem., 47, 68 (1982). 3. De door Barton e.a. gegeven verklaring voor de observaties, dat O-alkyl- -S-alkyl-dithiocarbonaten reageren met N^-dimethylhydrazine onder vor- ming van ^-alkyl-thiocarbamaten en ^-alkyl-thiocarbazaten, terwijl al- koxythiocarbonylimidazolen uitsluitend £-alkyl-thiocarbazaten geven, is hoogst twijfelachtig. -
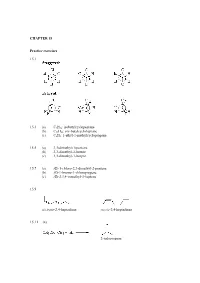
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

The Synthesis and Characterisation of a New Tröger's Base Content
Sys Rev Pharm 2020;11(7):319-323 A multifaceted review journal in the field of pharmacy The Synthesis and Characterisation of a New Tröger’s Base Content Methoxy Group Sadiq A. Karim1, Mohammed H. Said2, Jinan A. Abd3, Asim A. Balakit4, Ayad F. Alkaim5 1,2,5Chemistry department, College of Science for women, University of Babylon, Iraq. 3Department of Laser physics, College of Science for women, University of Babylon, Iraq. 4Pharmaceutical Chemistry department, College of Pharmacy, University of Babylon, Iraq. Corresponding author: [email protected] ABSTRACT Five Tröger’s base (TB) molecules were synthesized by reaction aniline’s Keywords: Tröger base, heterocyclic compounds, chirality derivatives which content a methoxy group with a supplement of methylene (dimethoxymethane (DMM)) in present trifluoroacetic acid (TFA) as a solvent Correspondence: and catalyst. This method afforded a good ratio product between 62% to 99%, Sadiq A. Karim1 all products were conforming by FTIR, HRMs, 1HNMR, 13CNMR, and XRD. 1,2,5Chemistry department, College of Science for women, University of Babylon, Iraq. Corresponding author: [email protected] INTRODUCTION collected by filtration and was dried in a vacuum oven at The first Tröger’s base was create in 1887 by Julius Tröger 50 °C for 2 h. during his Ph.D. studied, this compound was called Tröger base 1 (TB1) which from the condensation of methanal 1- Synthesis of 2,9-Dimethoxy-6H,12H-5,11- with 4-aminotoluene in HCl-catalysed media.1 The TB1 methanodibenzo[b,f](1,5)diazocine (TB-OCH3-1) (2,8-dimethyl-6H,12H-5,11- General procedure was followed using m-anisidine (9.1 methanodibenzo[b,f][1,5]diazocine) was conformed ml, 10.00 g, 81.20 mmol), DMM (10.8 ml, 9.269 g, 121.80 structure through chemical reaction by M. -

Aerobic Cometabolism of Chlorinated Aliphatic Hydrocarbons by Subsurface Microbes Grown on Methane, Propane and Butane from the Mcclellan Air Force Base
AN ABSTRACT OF THE THESIS OF Adisorn Tovanabootr for the degree of Masters of Science in Civil Engineering presented on April 23, 1997. Title: Aerobic Cometabolism of Chlorinated Aliphatic Hydrocarbons by Subsurface Microbes Grown on Methane, Propane and Butane from the McClellan Air Force Base. Abstract approved: Lewis Semprini Subsurface microorganisms from the McClellan Air Force Base were grown in batch aquifer microcosms on methane, propane, and butane as gaseous cometabolic substrates. The potential for aerobic cometabolism of chlorinated aliphatic hydrocarbons including trichloroethylene (TCE), 1,1,1-trichloroethane (1,1,1-TCA), and chloroform (CF) was determined. Stimulation of microorganisms on all of the substrates tested indicates a diverse microbial community exists in the McClellan subsurface. Indigenous methane and propane-utilizers were capable of transforming TCE and CF. Propane- utilizers very effectively transformed TCA, while methane-utilizers could not transform 1,1,1-TCA. The butane-utilizers were not able to degrade any of the CAHs tested. TCE was transformed most rapidly during the period of active methane consumption, and continued at a slower rate for about 1 weeks after methane was consumed. The propane culture remained active for up to four weeks after propane was consumed, and the rate followed first order kinetics. Different TCE transformation yields (Tv) (mg CAH/mg substrate) developed in replicate microcosms with time. Changes in TCE transformation ability resulted from changes in TCE concentration or TCE product toxicity. Both methane and propane-utilizers showed a positive correlation between initial TCE transformation rates and primary substrate utilization rates. The ratio of the zero order TCE transformation rate to primary substrate utilization rate was directly proportional to the ultimate transformation yield. -

On the Road to Carbene and Carbyne Complexes
ON THE ROAD TO CARBENE AND CARBYNE COMPLEXES Nobel Lecture, 11 December 1973 by ERNST OTTO FISCHER Inorganic Chemistry Laboratory, Technical University, Munich, Federal Republic of Germany Translation from the German text INTRODUCTION In the year 1960, I had the honour of giving a talk at this university* about sandwich complexes on which we were working at that time. I think I do not have to repeat the results of those investigations today. I would like to talk instead about a field of research in which we have been intensely interested in recent years: namely, the field of carbene complexes and, more recently, carbyne complexes. If we substitute one of the hydrogen atoms in a hydrocarbon of the alkane type - for example, ethane - by a metal atom, which can of course bind many more ligands, we arrive at an organometallic compound in which the organic radical is bound to the metal atom by a σ-bond (Fig. la). The earliest compounds of this kind were prepared more than a hundred years ago; the first was cacodyl, prepared by R. Bunsen (1), and then zinc dialkyls were prepared by E. Frankland (2). Later V. Grignard was able to synthesise alkyl magnesium halides by treating magnesium with alkyl halides (3). Grignard was awarded the Nobel Prize in 1912 for this effort. We may further recall the organo-aluminium compounds (4) of K. Ziegler which form the basis for the low pressure polymerisation, for example of ethylene. Ziegler and G. Natta were together honoured with the Nobel Prize in 1963 for their work on organometallic compounds.