Flammables CAS DOT SHHC Sources Number Chemical Name
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanistic Insights Into the Hydrocyanation Reaction
Mechanistic insights into the hydrocyanation reaction Citation for published version (APA): Bini, L. (2009). Mechanistic insights into the hydrocyanation reaction. Technische Universiteit Eindhoven. https://doi.org/10.6100/IR644067 DOI: 10.6100/IR644067 Document status and date: Published: 01/01/2009 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Numerical Simulation of a Two-Phase Flow for the Acrylonitrile Electrolytic Adiponitrile Process in a Vertical/Horizontal Electrolysis Cell
energies Article Numerical Simulation of a Two-Phase Flow for the Acrylonitrile Electrolytic Adiponitrile Process in a Vertical/Horizontal Electrolysis Cell Jiin-Yuh Jang * and Yu-Feng Gan Department of Mechanical Engineering, National Cheng-Kung University, Tainan 70101, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-6-2088573 Received: 7 September 2018; Accepted: 6 October 2018; Published: 12 October 2018 Abstract: This paper investigated the effect of oxygen holdup on the current density distribution over the electrode of a vertical/horizontal electrolysis cell with a two-dimensional Eulerian–Eulerian two-phase flow model in the acrylonitrile (AN) electrolytic adiponitrile (ADN) process. The physical models consisted of a vertical/horizontal electrolysis cell 10 mm wide and 600 mm long. The electrical potential difference between the anode and cathode was fixed at 5 V, which corresponded to a uniform current density j = 0.4 A/cm2 without any bubbles released from the electrodes. The effects of different inlet electrolyte velocities (vin = 0.4, 0.6, 1.0 and 1.5 m/s) on the void fraction and the current density distributions were discussed in detail. It is shown that, for a given applied voltage, as the electrolyte velocity is increased, the gas diffusion layer thickness decreased and this resulted in the decrease of the gas void fraction and increase of the corresponding current density; for a given velocity, the current density for a vertical cell was higher than that for a horizontal cell. Furthermore, assuming the release of uniform mass flux for the oxygen results in overestimation of the total gas accumulation mass flow rate by 2.8% and 5.8% and it will also result in underestimation of the current density by 0.3% and 2.4% for a vertical cell and a horizontal cell, respectively. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter
EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter EPA 454/B-18-008 August 2018 EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter U.S. Environmental Protection Agency Office of Air Quality Planning and Standards Air Quality Assessment Division Research Triangle Park, NC EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter 9/1/2018 Informational Document This informational document describes the emerging technologies that can measure and/or identify pollutants using state of the science techniques Forward Optical Remote Sensing (ORS) technologies have been available since the late 1980s. In the early days of this technology, there were many who saw the potential of these new instruments for environmental measurements and how this technology could be integrated into emissions and ambient air monitoring for the measurement of flux. However, the monitoring community did not embrace ORS as quickly as anticipated. Several factors contributing to delayed ORS use were: • Cost: The cost of these instruments made it prohibitive to purchase, operate and maintain. • Utility: Since these instruments were perceived as “black boxes.” Many instrument specialists were wary of how they worked and how the instruments generated the values. • Ease of use: Many of the early instruments required a well-trained spectroscopist who would have to spend a large amount of time to setup, operate, collect, validate and verify the data. • Data Utilization: Results from path integrated units were different from point source data which presented challenges for data use and interpretation. -

Borneol - Camphor - Isoborneol
EXP. 35 A & B OXIDATION-REDUCTION SCHEME: BORNEOL - CAMPHOR - ISOBORNEOL LEARNING OBJECTIVES: To illustrate the concepts of oxidation and reduction in organic chemistry, to illustrate the stereochemical effects of these reactions in certain systems, to use IR spectroscopy to characterize diastereomers and monitor reactions. QUIZ PREPARATION: Recitation notes and readings assigned in the syllabus. OXIDATION AND REDUCTION IN ORGANIC CHEMISTRY The concepts of oxidation and reduction in chemistry are fundamentally related to the loss and gain of electrons, respectively. For a specific atom, oxidation brings about an increase in the oxidation number, whereas reduction does the opposite. This is of course true in organic reactions as well, but it is frequently less clear where the gain or loss of electrons is taking place. For this reason, the concepts of oxidation and reduction are addressed from a different perspective in organic chemistry. Since carbon is the most important element in organic chemistry, we can define oxidation as a process whereby carbon gains bonds to more electronegative atoms. These can be any atoms more electronegative than carbon, such as chlorine or sulphur, but the most commonly seen in organic oxidations is oxygen. The more bonds to oxygen, the higher the oxidation state of the carbon involved. Examples of oxidation reactions are those that convert alkanes into alcohols, or alcohols into carbonyl compounds. The functional groups that contain the most highly oxidized form of carbon in organic compounds are the carboxylic acids and their derivatives. Reduction is the opposite of oxidation. We can define reduction as a process whereby carbon adds bonds to less electronegative atoms. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

1 Transformer Oil 2 Residual Fuel Oil 3 Diesel Oil Heavy 4
1 TRANSFORMER OIL 2 RESIDUAL FUEL OIL 3 DIESEL OIL HEAVY 4 SPINDLE OIL MIXTURES CONTAINING 5 CRUDE OIL 6 MOTOR OIL 7 ROAD OIL 8 FUEL OIL NO.4 9 FUEL OIL NO.5 10 FUEL OIL NO.6 LUBRICATING OILS AND 11 BLENDING STOCKS 12 PENETRATING OIL 13 TURBINE OIL 3 14 ROOFERS FLUX 15 CRUDE OIL 16 STRAIGHT RUN RESIDUE OCTAMETHYLCYCLOTETRA 17 SILOXANE BENZENEPROPANOIC ACID, 3,5- 1,1- 3,5-BIS(1,1-DIMETHYLETHYL 18 4- ), -C7-C9 4-HYDROXY-C7-C9ALCOHOL S BRANCHED AND LINEAR 19 1- -1- 1-PHENYL-1-XYLYL ETHANE BENZENETRICARBOXYLIC 20 ACID, TRIOCTYL ESTER 21 CASTOR OIL IMIDAZOLIUM COMPOUNDS, 1- -4,5- -1- 1-BENZYL-4,5-DIHYDRO-1-(H 22 -2- YDROXYETHYL)-2-NORCOC O ALKYL, CHLORIDES 4 2-PROPENOIC ACID 2- POLYMER WITH 4- 1,1- 4-(1,1-DIMETHYLETHYL)PHE 2,5- NOL,FORMALDEHYDE, 23 2- 2,5-FURANDIONE, 65% / 2-METHYLOXIRANE AND OXIRANE (65% IN NAPHTHA/XYLENE) 24 n-PENTYL PROPIONATE 25 -2- 2-ETHYLHEXYL ACRYLATE 26 DECYL ACRYLATE 27 RAPESEED OIL RAPESEED OIL (low erucic acid 4% 28 containing less than 4% free fatty acids) RAPE SEED OIL FATTY ACID 29 METHYL ESTERS ALCOHOLS (C12-C13), C12-C13 30 PRIMARY, LINEAR AND ESSENTIALLY LINEAR 31 C13+ ALCOHOLS (C13+) 5 ALCOHOLS (C14-C18), C14-C18 32 PRIMARY, LINEAR AND ESSENTIALLY LINEAR ALCOHOLS (C8-C11), C8-C11 33 PRIMARY, LINEAR AND ESSENTIALLY LINEAR ACID OIL MIXTURE FROM SOYABEAN, CORN (maize) 34 AND SUNFLOWER OIL REFINING NAPHTHALENE CRUDE 35 (MOLTEN) 36 SOYABEAN OIL SOYBEAN OIL FATTY ACID 37 METHYL ESTER 38 BUTYLBENZENE (all isomers) 39 TALLOW 40 TALLOW FATTY ACID (2- BIS(2-ETHYLHEXYL) 41 ) TEREPHTHALATE 42 -(2- ) DI-(2-ETHYLHEXYL) -

The Detection and Determination of Esters
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1958 The etD ection and Determination of Esters. Mohd. Mohsin Qureshi Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Qureshi, Mohd. Mohsin, "The eD tection and Determination of Esters." (1958). LSU Historical Dissertations and Theses. 501. https://digitalcommons.lsu.edu/gradschool_disstheses/501 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. Copright by Mohcl Mohsin Qureshi 1959 THE DETECTION AND DETERMINATION OF ESTERS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Mohd. Mohsin Qureshi M.Sc., Aligarh University, 1944 August, 1958 ACKNOWLEDGMENT The author wishes to express his sincere apprecia tion and gratitude to Dr. Philip W. West under whose guidance this research was carried out. He is grateful to Dr. James G. Traynham for sup plying him with a number of esters and for his many helpful suggestions. The financial support given to him by the Continental Oil Company is gratefully acknowledged. He offers his sincere thanks to Miss Magdalena Usategul for her valuable suggestions and her ungrudging help during the course of this investigation. Dr. Anil K. -

Preparation and Purification of Atmospherically Relevant Α
Atmos. Chem. Phys., 20, 4241–4254, 2020 https://doi.org/10.5194/acp-20-4241-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Technical note: Preparation and purification of atmospherically relevant α-hydroxynitrate esters of monoterpenes Elena Ali McKnight, Nicole P. Kretekos, Demi Owusu, and Rebecca Lyn LaLonde Chemistry Department, Reed College, Portland, OR 97202, USA Correspondence: Rebecca Lyn LaLonde ([email protected]) Received: 31 July 2019 – Discussion started: 6 August 2019 Revised: 8 December 2019 – Accepted: 20 January 2020 – Published: 9 April 2020 Abstract. Organic nitrate esters are key products of terpene oxidation in the atmosphere. We report here the preparation and purification of nine nitrate esters derived from (C)-3- carene, limonene, α-pinene, β-pinene and perillic alcohol. The availability of these compounds will enable detailed investigations into the structure–reactivity relationships of aerosol formation and processing and will allow individual investigations into aqueous-phase reactions of organic nitrate esters. Figure 1. Two hydroxynitrate esters with available spectral data. Relative stereochemistry is undefined. 1 Introduction derived ON is difficult, particularly due to partitioning into the aerosol phase in which hydrolysis and other reactivity Biogenic volatile organic compound (BVOC) emissions ac- can occur (Bleier and Elrod, 2013; Rindelaub et al., 2014, count for ∼ 88 % of non-methane VOC emissions. Of the to- 2015; Romonosky et al., 2015; Thomas et al., 2016). Hydrol- tal BVOC estimated by the Model of Emission of Gases and ysis reactions of nitrate esters of isoprene have been stud- Aerosols from Nature version 2.1 (MEGAN2.1), isoprene is ied directly (Jacobs et al., 2014) and the hydrolysis of ON estimated to comprise half, and methanol, ethanol, acetalde- has been studied in bulk (Baker and Easty, 1950). -
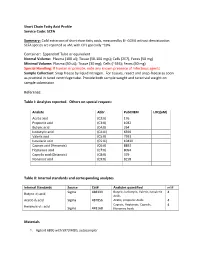
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Aldrich Raman
Aldrich Raman Library Listing – 14,033 spectra This library represents the most comprehensive collection of FT-Raman spectral references available. It contains many common chemicals found in the Aldrich Handbook of Fine Chemicals. To create the Aldrich Raman Condensed Phase Library, 14,033 compounds found in the Aldrich Collection of FT-IR Spectra Edition II Library were excited with an Nd:YVO4 laser (1064 nm) using laser powers between 400 - 600 mW, measured at the sample. A Thermo FT-Raman spectrometer (with a Ge detector) was used to collect the Raman spectra. The spectra were saved in Raman Shift format. Aldrich Raman Index Compound Name Index Compound Name 4803 ((1R)-(ENDO,ANTI))-(+)-3- 4246 (+)-3-ISOPROPYL-7A- BROMOCAMPHOR-8- SULFONIC METHYLTETRAHYDRO- ACID, AMMONIUM SALT PYRROLO(2,1-B)OXAZOL-5(6H)- 2207 ((1R)-ENDO)-(+)-3- ONE, BROMOCAMPHOR, 98% 12568 (+)-4-CHOLESTEN-3-ONE, 98% 4804 ((1S)-(ENDO,ANTI))-(-)-3- 3774 (+)-5,6-O-CYCLOHEXYLIDENE-L- BROMOCAMPHOR-8- SULFONIC ASCORBIC ACID, 98% ACID, AMMONIUM SALT 11632 (+)-5-BROMO-2'-DEOXYURIDINE, 2208 ((1S)-ENDO)-(-)-3- 97% BROMOCAMPHOR, 98% 11634 (+)-5-FLUORODEOXYURIDINE, 769 ((1S)-ENDO)-(-)-BORNEOL, 99% 98+% 13454 ((2S,3S)-(+)- 11633 (+)-5-IODO-2'-DEOXYURIDINE, 98% BIS(DIPHENYLPHOSPHINO)- 4228 (+)-6-AMINOPENICILLANIC ACID, BUTANE)(N3-ALLYL)PD(II) CL04, 96% 97 8167 (+)-6-METHOXY-ALPHA-METHYL- 10297 ((3- 2- NAPHTHALENEACETIC ACID, DIMETHYLAMINO)PROPYL)TRIPH 98% ENYL- PHOSPHONIUM BROMIDE, 12586 (+)-ANDROSTA-1,4-DIENE-3,17- 99% DIONE, 98% 13458 ((R)-(+)-2,2'- 963 (+)-ARABINOGALACTAN BIS(DIPHENYLPHOSPHINO)-1,1'-