Technical Report for the Substantial Modification Rule for 62-730, F.A.C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(A) 1) at First a Pair of Each 10G. of Sample Was Extracted with Ether by the Soxhlet's Apparatus for a Week
52 [vol. 6, THE DISTRIBUTION OF PHOSPHORUS IN COW'S MILK AND THE SCHEME FOR THE SEPARATION OF PHOSPHATIDES By Rinjiro SASAKI (From the Institute of Agricultural Chemistry, College of Agriculture, Tokyo Imperial University) (Received 9th September 1930) A. The Distribution of Phosphorus in Cow's milk In order to extract the phosphatides, it is necessary first to determine the distribution of phosphorus in cow's milk. Liquid milk can not be extracted with ether or with other organic solvents owing to its large content of water. The substance applied in this experiment is the milk powder, dried by the Buflovak drum drier below 70•Ž. Its content of moisture is 4.498%, deter mined by the method of drying in the air bath of 105•Ž. The content of total phosphorus was determined(5) in the ash of sample which was burned with a little of fusing mixture. In 100g. of milk powder In 100g. of dry matter Total phosphorus 0.745g. 0.781g. The amounts of phosphorus, which are soluble in the various organic sol vents, were determined by extracting with three solvents in the following orders. (A) Ether-Acetone-Alcohol (B) Acetone-Ether-Alcohol (C) Alcohol-Ether-Acetone Experiment (A) 1) At first a pair of each 10g. of sample was extracted with ether by the Soxhlet's apparatus for a week. After the extraction had been comp leted, the extract was freed from ether and weighed. In 100g.of milkpowder In 100g.of dry matter Total ether matters soluble 3.609g. 3.779g. 2) The above extract was dissolved in a very little of ether. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Chromatographic Separation of Alkaline Earth Metals Using Alpha-Hydroxyisobutyric Acid
AN ABSTRACT OF THE THESIS OF JOHN ARTHUR HAUSCHILD for the MASTER OF SCIENCE (Name) (Degree) in CHEMISTRY (ANALYTICAL) presented on (Major) Title: CHROMATOGRAPHIC SEPARATION OF ALKALINE EARTH METALS USING ALPHA-HYDROXYISOBUYRIC ACID Abstract approved: Redacted for Privacy Max B. Williams A systematic study of the elution of magnesium and calcium from Dowex 50 X 8 resin using a-hydroxyisobutyric acid (a-HIBA) at various pH values and concentrations, indicated that the difference in the equilibrium distribution coefficients of these two elements was large enough for a good separation.This fact was applied to develop a chromatographic procedure for the separationof milligram quantities of magnesium, calcium, strontium, and barium.After magnesium was eluted with 0. 22M a-HIBA at pH 4. 5, thethree remaining elements were eluted by varying the concentration and pH of a-HIBAduring the course of the elution (exponential gradient elution).After its respec- tive elution, each alkaline earth metal was directly determined by atomic absorption spectroscopy.Using this method, several success- ful analyses of synthetic samples (similar to the composition of sea water) were performed.Yield determinations of the alkaline earth metals from these analyses were consistently greater than 93%, with the overall average yield being 98%. Chromatographic Separation of Alkaline Earth Metals Using Alpha-Hydroxyisobutyric Acid by John Arthur Haus child A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master -

Phosphate-Based Treatments for Conservation of Stone
RILEM Technical Letters (2017) 2: 14‐19 DOI: http://dx.doi.org/10.21809/rilemtechlett.2017.34 Phosphate‐based treatments for conservation of stone Enrico Sassoni a* a Department of Civil, Chemical, Environmental and Materials Engineering, University of Bologna, Via Terracini 28, 40131, Bologna, Italy Received: 30 May 2017 / Accepted: 09 August 2017 / Published online: 9 October 2017 © The Author(s) 2017. This article is published with open access and licensed under a Creative Commons Attribution 4.0 International License. Abstract To overcome the limitations of currently available protectives and consolidants for carbonate stones (such as marble and limestone), in 2011 the use of calcium phosphate was proposed. The idea is forming calcium phosphates (ideally hydroxyapatite) as the reaction product between the substrate and an aqueous solution of a phosphate salt that the stone is treated with. In this paper, the studies aimed at identifying the best treatment conditions (in terms of nature and concentration of the phosphate precursor, solution pH, reaction time, ionic and organic additions) are first briefly summarized. Then, the efficacy of the phosphate treatment in protecting marble from dissolution in rain and restoring cohesion of weathered marble and limestone is discussed. Some recent studies on the use of the phosphate treatment on alternative substrates and some future steps for research on the topic are finally outlined. Keywords: Cultural heritage; Marble; Hydroxyapatite; Protection; Consolidation 1 Introduction improve mechanical properties, by providing a binding action between the stone grains. Organic products are A great part of cultural heritage objects (e.g. monuments, effective in improving mechanical properties, but again architectural decorations and statues) is made of carbonate they lack compatibility and durability. -

United States Patent Office Patented Apr
3,030,421 United States Patent Office Patented Apr. 17, 1962 2 3,030,421 perature and under a reduced pressure. In the case of PROCESS FOR PREPARNG TRHYDROXY most of the catalysts the reaction products, which are free METHYL-PHOSPHINE s from solvents, solidify already when being cooled to room Martin Reuter and Ludwig Ortane, Frankfurt an Main, temperature. If, in the case of some catalysts, they Germany, assignors to Farbwerke Hoechst Aktienger 5 solidify only at a lower temperature and still contain to sellschaft vormas Meister Lucius & Briining, Frank a greater extent oily by-products and/or phosphonium furt am Main, Germany, a corporation of Gerinally hydroxide, they can be separated from the latter by filter No Drawing. Fied Jaa. 14, 1958, Ser. No. 708,764 ing or pressing. - - - Claims priority, application Germany Jan. 23, 1957 It is to be assumed that the crystalline main product 6 Canas. (C. 260-606.5) O of the present invention constitutes the hitherto unknown We have found that a new and valuable phosphorus trihydroxymethyl-phosphine. Main and by-products are compound carrying hydroxymethyl groups at the phos easily soluble in water and methanol and sparingly solu phorus atom can be prepared by reacting 1 mol of formal ble in fat dissolvers. dehyde with 4 mol of phosphine, preferably in the pres The reaction products of the invention can be used as ence of water, and in the presence of Small quantities of 5 insecticides, additives for lubricants, flame-proofing agents finely distributed metals that do not belong to the alkali for wood and textiles and as intermediates for these sub metals or alkaline earth metals and/or their compounds Staces. -

1,45%,562 UNITED SATES P All.‘ Bl If" Til?
Patented May 1, 1923. 1,45%,562 UNITED SATES P All.‘ bl if" til? . ROBERT E. WILSON, LEON ‘JV. PARSONS, AND STANLEY 1E. OHXSHOLIYI, OF WASHINGTON, DISTRICT OF COLUMBIA. PROCESS THE PRODUCTION OE‘ All‘HALLEAETELMLETAL PERTJIANGANATES. N0 Drawing". Application ?led September 27, 1918. Serial No. 255,975. To (all to]: am it may concern. .' manganate by oxidation or acidification, Be it known that we, Romain‘ E. lllinsou, metatheses into calcium pern'iangamite by LEON “7. Parsons, and STANLEY L. Unis treatment With calcium sulphate or milk at HoLM, citizens of the United States. and sta lime. tioned at ViTashington, District of Columbia, O'li' these four possible methods, (1.) is not 60 in the o?icc of the Director the Chemical a possible large scale method. on account l/Varfare Service, Research Division, have in of its use ot silver; (2) and are elec vented a Process for the ll’roduction oif Al trolytic methods Without a. great deal out kali-Earth-l\letal Permanpjanates, of which promise, and are to be considered elsewhere; ll) the ‘following is a speci?cation. (ll) the principal subject of this applica G3 in The present invention relates to the pro tion. duction oi? alkah» earth metal permangam Three distinct methods for preparing: ba~ nates and especially the permanganates of rium (or strontium) manganate have been calcium and magnesium as these have beenv here investigated. The ?rst of? these meth found to be very ellicient oxidizing agents ods involves heating together barium perox 70 for certain purposes, more e?icient even. than ide, hydroxide, or a salt, such as the nitrate the permanganates of the allmliearth metals. -

Status of Industry Efforts to Replace Halon Fire Extinguishing Agents
STATUS OF INDUSTRY EFFORTS TO REPLACE HALON FIRE EXTINGUISHING AGENTS Robert T. Wickham, P.E. March 16, 2002 WICKHAM ASSOCIATES NOTICE This document is disseminated under the sponsorship of the U.S. Environmental Protection Agency in the interest of information exchange. The views expressed in this report are those of the author and do not necessarily reflect those of the US Environmental Protection Agency. The US Environmental Protection Agency does not assume liability for the contents or use thereof and further the Agency does not endorse products or manufacturers. Trade or manufacturer's names appear herein solely because they are considered essential to the objective of this report. The following commercial products (requiring a trademark designation ® or ™) are mentioned in this report. Due to the frequency of usage, trademarks are not indicated. Mention of a product does not constitute endorsement or rejection of the product. Ansul FE-25 Argonite FE-36 Argotec FM-200 CEA-410 Halotron I CEA-614 Inergen CEA-308 NAF P-IV Envirogel NAF S-III FE-13 NN100 FE-227 Triodide FE-241 There are no restrictions on copying or distribution of this document. Additional copies of this report are available from ….. Wickham Associates 9 Winding Brook Drive Stratham, New Hampshire 03885 USA Tel: +603-772-3229 Fax: +603-772-7305 email: [email protected] The report is available at http://home.attbi.com/~wickham/downloads.htm for downloading in Adobe Acrobat portable document format (pdf). Preface This report is not intended to be a market study, but instead a snapshot of the progress industry and the government are making in employing non ozone depleting alternatives to halons in the fire protection sector. -

Hydrothermal Synthesis of Molybdenum Based Oxides for The
Hydrothermal synthesis of molybdenum based oxides for the application in catalysis Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) Fakultät für Chemie und Biowissenschaften Karlsruher Institut für Technologie (KIT) - Universitätsbereich genehmigte DISSERTATION von Dipl.-Ing. (FH) Kirsten Schuh aus Mainz Dekan: Prof. Dr. Peter Roesky Referent: Prof. Dr. Jan-Dierk Grunwaldt Korreferent: Prof. Dr. Anker Degn Jensen Tag der mündlichen Prüfung: 17. April 2014 Acknowledgements Acknowledgements I owe many thanks to a lot of people who have helped, supported and encouraged me during my doctoral studies, not just scientifically but also personally. First I would like to thank my supervisor Prof. Dr. Jan-Dierk Grunwaldt for the opportunity to complete my doctoral studies in his group and for providing me with a very interesting and diversified topic. I am grateful for the scientific freedom he gave me, the possibility to spend several months at the Technical University of Denmark as well as University of Zurich and for the opportunity to attend international conferences. I am grateful to Dr. Wolfgang Kleist for his scientific help especially with presentations and publications making the manuscripts reader friendly. I would also like to thank Prof. Dr. Anker Degn Jensen for agreeing to be my co- supervisor, for very helpful corrections and suggestions of abstracts, manuscripts and presentations and for giving me the opportunity to spend four months in his group at the Technical University of Denmark (DTU), where I felt very welcome. I am especially grateful for the help of Dr. Martin Høj, who put the selective oxidation set- up at DTU into operation, tested several of my samples for selective oxidation of propylene and performed TEM measurements of my FSP samples. -

Fine Biocompatible Powders Synthesized from Calcium Lactate and Ammonium Sulfate
ceramics Article Fine Biocompatible Powders Synthesized from Calcium Lactate and Ammonium Sulfate Maksim Kaimonov 1,* , Tatiana Shatalova 1,2 , Yaroslav Filippov 1,3 and Tatiana Safronova 1,2 1 Department of Materials Science, Lomonosov Moscow State University, Building, 73, Leninskie Gory, 1, 119991 Moscow, Russia; [email protected] (T.S.); fi[email protected] (Y.F.); [email protected] (T.S.) 2 Department of Chemistry, Lomonosov Moscow State University, Building, 3, Leninskie Gory, 1, 119991 Moscow, Russia 3 Research Institute of Mechanics, Lomonosov Moscow State University, Michurinsky pr., 1, 119192 Moscow, Russia * Correspondence: [email protected]; Tel.: +7-952-889-11-43 Abstract: Fine biocompatible powders with different phase compositions were obtained from a 0.5 M solution of ammonium sulfate (NH4)2SO4 and calcium lactate Ca(C3H5O3)2. The powder ◦ after synthesis and drying at 40 C included calcium sulfate dehydrate CaSO4·2H2O and calcite ◦ CaCO3. The powder after heat treatment at 350 C included β-hemihydrate calcium sulfate β- CaSO4·0.5H2O, γ-anhydrite calcium sulfate γ-CaSO4 and calcite CaCO3. The phase composition of ◦ powder heat-treated at 600 C was presented as β-anhydrate calcium sulfate β-CaSO4 and calcite ◦ CaCO3. Increasing the temperature up to 800 C leads to the sintering of a calcium sulfate powder β β consisting of -anhydrite calcium sulfate -CaSO4 main phase and a tiny amount of calcium oxide CaO. The obtained fine biocompatible powders of calcium sulfate both after synthesis and after heat Citation: Kaimonov, M.; Shatalova, treatment at temperature not above 600 ◦C can be recommended as a filler for producing unique T.; Filippov, Y.; Safronova, T. -

Chemistry Inventory; Fall
CHEMISTRY FALL 2005 MSDS Mfg.'s Name Chemical Name Quantity Stored Storage Conditions (on file = 9) Aluminum 9 1.5 kg Aluminum chloride, anhydrous, 98.5% 9 0.2 kg Aluminum chloride · 6H2O 9 0.5 kg Aluminum hydroxide 9 0.5 kg Aluminum nitrate 9 0.5 kg Aluminum sulfate 9 0.5 kg Ammonia, concentrated 9 4.0 L Ammonium acetate 9 0.2 kg Ammonium chloride 9 Ammonium dihydrogen phosphate (monobasic) 9 0.4 kg J.T. Baker Ammonium hydrogen phosphate (dibasic) No 0.5 kg Ammonium nitrate 9 2.5 kg Ammonium oxalate 9 0.7 kg Ammonium peroxydisulfate 9 0.5 kg Ammonium sulfate 9 0.2 kg Antimony 9 0.4 kg Barium chloride, anhydrous 9 2.5 kg Barium chloride · 2H2O 9 2.5 kg Barium nitrate 9 0.8 kg Bismuth 9 2.0 kg Boric Acid 9 0.4 kg Brass 9 Bromine 9 2.5 kg Cadmium 9 0.1 kg Cadmium nitrate 9 0.3 kg Calcium acetate · xH2O 9 0.5 kg Calcium carbide 9 1.0 kg Calcium carbonate 9 2.2 kg Calcium chloride 9 1.0 kg Calcium hydroxide 9 0.3 kg Calcium nitrate · 4H2O 9 1.0 kg Calcium oxide 9 0.3 kg Calcium sulfate · 2H2O 9 1.0 kg Carbon 9 0.1 kg Ceric ammonium nitrate 9 0.5 kg Cesium chloride 9 0.01 kg Chromium 9 0.01 kg Chromium chloride 9 0.5 kg Chromium nitrate 9 0.5 kg Cobalt 9 0.025 kg Cobalt chloride 9 0.7 kg Cobalt nitrate 9 0.6 kg Copper (assorted) 9 4.0 kg Copper acetate 9 0.05 kg Copper chloride 9 0.1 kg Copper nitrate 9 3.5 kg Copper oxide 9 0.4 kg Cupric sulfate, anhydrous 9 0.5 kg Cupric sulfate · 5H2O 9 2.75 kg EDTA 9 0.6 kg Iodine 9 2.0 kg Iron (assorted) 9 5.0 kg MSDS Mfg.'s Name Chemical Name Quantity Stored Storage Conditions (on file = 9) Ferric ammonium -
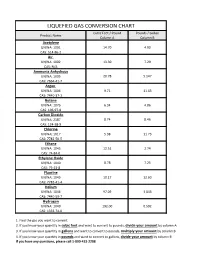
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL