Example Exercise 7.1 Classifying Compounds and Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Naming Polyatomic Ions and Acids Oxyanions
Oxyanions Naming Polyatomic Ions and Oxyanions Acids Oxyanions- negative ions containing Oxyanions may contain the prefix oxygen. “hypo-”, less than, or “per-”, more than. These have the suffix “-ate” or “-ite” For example - “-ate” means it has more oxygen atoms ClO4 Perchlorate bonded, “-ite” has less - ClO3 Chlorate For example - ClO2 Chlorite 2- SO4 sulfate ClO- Hypochlorite 2- SO3 sulfite Acids Naming acids Naming Acids Certain compounds produce H+ ions in Does it contain oxygen? water, these are called acids. If it does not, it gets the prefix “hydro-” and If it does contain an oxyanion, then the suffix “-ic acid” replace the ending. You can recognize them because the neutral compound starts with “H”. HCl If the ending was “–ate”, add “-ic acid” Hydrochloric acid If the ending was “–ite”, add “-ous acid” For example HCl, H2SO4, and HNO3. HF Don’t confuse a polyatomic ion with a H2SO4 Sulfuric Acid Hydrofluoric acid neutral compound. H2SO3 Sulfurous Acid HCN HCO - is hydrogen carbonate, not an acid. 3 Hydrocyanic acid Examples Examples Nomenclature (naming) of Covalent compounds HNO3 HNO3 Nitric Acid HI HI Hydroiodic acid H3AsO4 H3AsO4 Arsenic Acid HClO2 HClO2 Chlorous Acid 1 Determining the type of bond Covalent bonding is very Covalent bonding is very First, determine if you have an ionic different from ionic naming compound or a covalent compound. similar to ionic naming A metal and a nonmetal will form an You always name the one that is least Ionic names ignored the subscript ionic bond. electronegative first (furthest from because there was only one possible Compounds with Polyatomic ions form fluorine) ratio of elements. -

Chemistry: the Molecular Nature of Matter and Change
REVISED CONFIRMING PAGES The Components of Matter 2.1 Elements, Compounds, and 2.5 The Atomic Theory Today 2.8 Formula, Name, and Mass of Mixtures: An Atomic Overview Structure of the Atom a Compound 2.2 The Observations That Led to Atomic Number, Mass Number, and Binary Ionic Compounds an Atomic View of Matter Atomic Symbol Compounds That Contain Polyatomic Mass Conservation Isotopes Ions Definite Composition Atomic Masses of the Elements Acid Names from Anion Names Multiple Proportions 2.6 Elements: A First Look at the Binary Covalent Compounds The Simplest Organic Compounds: Dalton’s Atomic Theory Periodic Table 2.3 Straight-Chain Alkanes Postulates of the Atomic Theory Organization of the Periodic Table Masses from a Chemical Formula How the Atomic Theory Explains Classifying the Elements Representing Molecules with a the Mass Laws Compounds: An Introduction 2.7 Formula and a Model to Bonding 2.4 The Observations That Led to Mixtures: Classification and the Nuclear Atom Model The Formation of Ionic Compounds 2.9 Separation Discovery of the Electron and The Formation of Covalent An Overview of the Components of Its Properties Compounds Matter Discovery of the Atomic Nucleus (a) (b) (right) ©Rudy Umans/Shutterstock IN THIS CHAPTER . We examine the properties and composition of matter on the macroscopic and atomic scales. By the end of this chapter, you should be able to • Relate the three types of matter—elements or elementary substances, compounds, and mixtures—to the simple chemical entities that they comprise—atoms, ions, and molecules; -

Pp-03-25-New Dots.Qxd 10/23/02 2:41 PM Page 778
pp-03-25-new dots.qxd 10/23/02 2:41 PM Page 778 778 PRAESODYMIUM PRAESODYMIUM [7440–10–0] Symbol Pr; atomic number 59; atomic weight 140.908; a lanthanide–series rare earth element; belongs to the cerium group of rare earths; electron con- figuration [Xe] 4f36s2; partially filled f subshell; valence states +3, +4; most 3+ stable oxidation state +3; electrode potential E°/V (aq) for Pr + 3e¯ ↔ Pr is –2.35 V; atomic radius 1.828 Å; first ionization potential 5.46 eV; one natu- rally–occurring isotope, Pr–141; twenty–nine artificial radioactive isotopes known in the mass range 124, 126–140 and 142–154; the longest–lived isotope Pr–143, t1/2 13.57 day, and the shortest–lived isotope Pr–124, t1/2 1.2 second. History, Occurrence, and Uses Mosander extracted from the mineral lanthana a rare earth fraction, named didymia in 1841. In 1879, Boisbaudran separated a rare earth oxide called samaria (samarium oxide) from the didymia fraction obtained from the mineral samarskite. Soon after that in 1885, Baron Auer von Welsbach iso- lated two other rare earths from didymia. He named them as praseodymia (green twin) and neodymia (new twin) after their source didymia (twin). The name praseodymium finally was assigned to this new element, derived from the two Greek words, prasios meaning green and didymos meaning twin. Praseodymium occurs in nature associated with other rare earths in a rel- atively high abundance. It is more abundant than some common metals such as silver, gold, or antimony. The average concentration of this metal in the earth’s crust is estimated to be 8.2 mg/kg. -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Acetate C2H3O2 Or CH3COO Or CH3CO2 Ammonium NH4
Name:_____________________ Memorization For AP Chem Polyatmic Ions AP Chemistry Polyatomic Ion list - - - Acetate C2H3O2 or CH 3COO or CH 3CO 2 + Ammonium NH 4 - Ammonium Carbonate NH 4CO 3 Cyanide CN - 2- Carbonate CO 3 2- Dichromate Cr 2O7 - Dihydrogen phosphate H2PO 4 - Hydrogen carbonate HCO 3 - Hydrogen sulfate HSO 4 - Hydronium H3O Hydroxide OH - 2- Monohydrogen Phosphate HPO 4 - Nitrate NO 3 - Nitrite NO 2 2- Oxalate C2O4 - Permanganate MnO 4 3- Phosphate PO 4 2- Sulfate SO 4 2- Sulfite SO 3 Hypochlorite ClO - - Chlorite ClO 2 - Chlorate ClO 3 - Perchlorate ClO 4 2- Chromate CrO 4 2- Silicate SiO 3 Thiocyanate SCN - 2- Thiosulfate S2O3 - Bromate BrO 3 2- Selenate SeO 4 - Bisulfite HSO 3 Name:_____________________ Memorization For AP Chem Rules for Naming an Acid When the name of the anion ends in –ide, the acid name begins with the prefix hydro-, the stem of the anion has the suffix –ic and it is followed by the word acid. -ide becomes hydro _____ic Acid Cl- is the Chloride ion so HCl = hydrochloric acid When the anion name ends in –ite, the acid name is the stem of the anion with the suffix –ous, followed by the word acid. -ite becomes ______ous Acid - ClO 2 is the Chlorite ion so HClO 2 = Chlorous acid. When the anion name ends in –ate, the acid name is the stem of the anion with the suffix –ic, followed by the word acid. -ate becomes ______ic Acid - ClO 3 is the Chlorate ion so HClO 3 = Chloric acid. Rules for Naming Ionic Compounds 1. -

WO 2014/029634 Al 27 February 2014 (27.02.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/029634 Al 27 February 2014 (27.02.2014) P O P C T (51) International Patent Classification: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, C09C 1/02 (2006.01) HN, HR, HU, ID, IL, ΓΝ , IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/EP20 13/066666 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 8 August 2013 (08.08.2013) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, 1218 1089.9 20 August 2012 (20.08.2012) EP UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 61/693,350 27 August 2012 (27.08.2012) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (71) Applicant: OMYA INTERNATIONAL AG [CH/CH]; MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, Baslerstrasse 42, CH-4665 Oftringen (CH). -

On the Solubility of Radium Sulfate and Carbonate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Chalmers Publication Library THESIS FOR THE DEGREE OF LICENTIATE OF ENGINEERING On the solubility of radium sulfate and carbonate Artem Vasilyevich Matyskin Nuclear Chemistry Department of Chemistry and Chemical Engineering CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden 2016 On the solubility of radium sulfate and carbonate © ARTEM VASILYEVICH MATYSKIN, 2016 Technical report number: 2016:04 ISSN number: 1652-943X Nuclear Chemistry Department of Chemistry and Chemical Engineering Chalmers University of Technology SE – 412 96 Göteborg Sweden Telephone +46(0)31-772 1000 Cover: Radium sulfate powder Chalmers Reproservice Gothenburg, Sweden 2016 On the solubility of radium sulfate and carbonate Artem V. Matyskin Nuclear Chemistry Department of Chemistry and Chemical Engineering Chalmers University of Technology Abstract Radium is one of the most toxic elements and its concentration in different human activities and migration from man-made wastes provokes a strong interest in environmental science. To be able to model the migration process, reliable experimental thermodynamic data of radium compounds are needed. In this work details of the safe radium source disassembly which were previously used in brachytherapy are described and different methods for conversion of RaSO4 into aqueous solution are reviewed. The method of choice included three cycles of RaSO4 heating in 1.5 M Na2CO3 up to 85 ºC, cooling and subsequent removal of supernatant. X-ray diffraction studies showed that the method allows the synthesis of amorphous RaCO3, which can be dissolved in mineral acid. Gamma spectrometric measurements showed that most of the initial RaSO4 was 210 converted into solution and that 7 ± 1 % of the initial Pb was co-precipitated with RaCO3. -
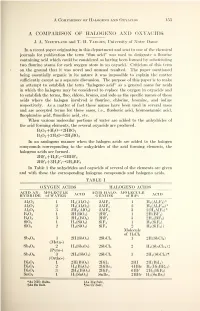
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

AAAS. the American Association for the Advancement of Science Was
A AAAS. The American Association for the Advancement of Science was movement through a planetary atmosphere to provide thermal protection founded in 1848 and incorporated in 1874. Its objectives are to further the to the underlying structure. See also Ablating Material. work of scientists, to facilitate cooperation among them, to foster scientific freedom and responsibility, to improve the effectiveness of science in ABRASION. All metallic and nonmetallic surfaces, no matter how promoting human welfare, to advance education in science, and to increase smooth, consist of minute serrations and ridges that induce a cutting or public understanding and appreciation for the importance and promise of tearing action when two surfaces in contact move with respect to each the methods of science in human progress. The AAAS head quarters is other. This wearing of the surfaces is termed abrasion. Undesirable abrasion in Washington, DC. Additional information on the AAAS can be found at may occur in bearings and other machine elements, but abrasion is also http://www.aaas.org/ and http://www.sciencemag.org/ . adapted to surface finishing and machining, where the material is too hard to be cut by other means, or where precision is a primary requisite. ABACA. The sclerenchyma bundles from the sheathing leaf bases of Temperature is a significant factor: friction may raise the temperature of Musa textilis, a plant closely resembling the edible banana plant. These the surface layers to the point where they become subject to chemical bundles are stripped by hand, after which they are cleaned by drawing over attack. Abrasion causes deterioration of many materials, especially of a rough knife.