Intravenous to Oral Conversion for Antimicrobials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200327 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number(s) 200327 Priority or Standard Standard Submit Date(s) December 29, 2009 Received Date(s) December 30, 2009 PDUFA Goal Date October 30, 2010 Division / Office Division of Anti-Infective and Ophthalmology Products Office of Antimicrobial Products Reviewer Name(s) Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD Review Completion October 29, 2010 Date Established Name Ceftaroline fosamil for injection (Proposed) Trade Name Teflaro Therapeutic Class Cephalosporin; ß-lactams Applicant Cerexa, Inc. Forest Laboratories, Inc. Formulation(s) 400 mg/vial and 600 mg/vial Intravenous Dosing Regimen 600 mg every 12 hours by IV infusion Indication(s) Acute Bacterial Skin and Skin Structure Infection (ABSSSI); Community-acquired Bacterial Pneumonia (CABP) Intended Population(s) Adults ≥ 18 years of age Template Version: March 6, 2009 Reference ID: 2857265 Clinical Review Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD NDA 200327: Teflaro (ceftaroline fosamil) Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ......................................... 9 1.1 Recommendation on Regulatory Action ........................................................... 10 1.2 Risk Benefit Assessment.................................................................................. 10 1.3 Recommendations for Postmarketing Risk Evaluation and Mitigation Strategies ........................................................................................................................ -

Ceftazidime for Injection) PHARMACY BULK PACKAGE – NOT for DIRECT INFUSION
PRESCRIBING INFORMATION FORTAZ® (ceftazidime for injection) PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION To reduce the development of drug-resistant bacteria and maintain the effectiveness of FORTAZ and other antibacterial drugs, FORTAZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Ceftazidime is a semisynthetic, broad-spectrum, beta-lactam antibacterial drug for parenteral administration. It is the pentahydrate of pyridinium, 1-[[7-[[(2-amino-4 thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1 azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, hydroxide, inner salt, [6R-[6α,7β(Z)]]. It has the following structure: The molecular formula is C22H32N6O12S2, representing a molecular weight of 636.6. FORTAZ is a sterile, dry-powdered mixture of ceftazidime pentahydrate and sodium carbonate. The sodium carbonate at a concentration of 118 mg/g of ceftazidime activity has been admixed to facilitate dissolution. The total sodium content of the mixture is approximately 54 mg (2.3 mEq)/g of ceftazidime activity. The Pharmacy Bulk Package vial contains 709 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3mEq) per gram of ceftazidime. FORTAZ in sterile crystalline form is supplied in Pharmacy Bulk Packages equivalent to 6g of anhydrous ceftazidime. The Pharmacy Bulk Package bottle is a container of sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous use. THE PHARMACY BULK PACKAGE IS NOT FOR DIRECT INFUSION, FURTHER DILUTION IS REQUIRED BEFORE USE. -

Cefoxitin Versus Piperacillin– Tazobactam As Surgical Antibiotic Prophylaxis in Patients Undergoing Pancreatoduodenectomy: Protocol for a Randomised Controlled Trial
Open access Protocol BMJ Open: first published as 10.1136/bmjopen-2020-048398 on 4 March 2021. Downloaded from Cefoxitin versus piperacillin– tazobactam as surgical antibiotic prophylaxis in patients undergoing pancreatoduodenectomy: protocol for a randomised controlled trial Nicole M Nevarez ,1 Brian C Brajcich,2 Jason Liu,2,3 Ryan Ellis,2 Clifford Y Ko,2 Henry A Pitt,4 Michael I D'Angelica,5 Adam C Yopp1 To cite: Nevarez NM, ABSTRACT Strengths and limitations of this study Brajcich BC, Liu J, et al. Introduction Although antibiotic prophylaxis is Cefoxitin versus piperacillin– established in reducing postoperative surgical site tazobactam as surgical ► A major strength of this study is the multi- infections (SSIs), the optimal antibiotic for prophylaxis in antibiotic prophylaxis institutional, double- arm, randomised controlled in patients undergoing pancreatoduodenectomy (PD) remains unclear. The study trial design. objective is to evaluate if administration of piperacillin– pancreatoduodenectomy: ► A limitation of this study is that all perioperative care protocol for a randomised tazobactam as antibiotic prophylaxis results in decreased is at the discretion of the operating surgeon and is controlled trial. BMJ Open 30- day SSI rate compared with cefoxitin in patients not standardised. 2021;11:e048398. doi:10.1136/ undergoing elective PD. ► All data will be collected through the American bmjopen-2020-048398 Methods and analysis This study will be a multi- College of Surgeons National Surgical Quality ► Prepublication history for institution, double- arm, non- blinded randomised controlled Improvement Program, which is a strength for its this paper is available online. superiority trial. Adults ≥18 years consented to undergo PD ease of use but a limitation due to the variety of data To view these files, please visit for all indications who present to institutions participating included. -

Activity of Aztreonam in Combination with Ceftazidime-Avibactam Against
Diagnostic Microbiology and Infectious Disease 99 (2021) 115227 Contents lists available at ScienceDirect Diagnostic Microbiology and Infectious Disease journal homepage: www.elsevier.com/locate/diagmicrobio Activity of aztreonam in combination with ceftazidime–avibactam against serine- and metallo-β-lactamase–producing ☆ ☆☆ ★ Pseudomonas aeruginosa , , Michelle Lee a, Taylor Abbey a, Mark Biagi b, Eric Wenzler a,⁎ a College of Pharmacy, University of Illinois at Chicago, Chicago, IL, USA b College of Pharmacy, University of Illinois at Chicago, Rockford, IL, USA article info abstract Article history: Existing data support the combination of aztreonam and ceftazidime–avibactam against serine-β-lactamase Received 29 June 2020 (SBL)– and metallo-β-lactamase (MBL)–producing Enterobacterales, although there is a paucity of data against Received in revised form 15 September 2020 SBL- and MBL-producing Pseudomonas aeruginosa. In this study, 5 SBL- and MBL-producing P. aeruginosa (1 Accepted 19 September 2020 IMP, 4 VIM) were evaluated against aztreonam and ceftazidime–avibactam alone and in combination via broth Available online xxxx microdilution and time-kill analyses. All 5 isolates were nonsusceptible to aztreonam, aztreonam–avibactam, – – Keywords: and ceftazidime avibactam. Combining aztreonam with ceftazidime avibactam at subinhibitory concentrations Metallo-β-lactamase produced synergy and restored bactericidal activity in 4/5 (80%) isolates tested. These results suggest that the VIM combination of aztreonam and ceftazidime–avibactam may be a viable treatment option against SBL- and IMP MBL-producing P. aeruginosa. Aztreonam © 2020 Elsevier Inc. All rights reserved. Ceftazidime–avibactam Synergy 1. Introduction of SBLs and MBLs harbored by P. aeruginosa are fundamentally different than those in Enterobacterales (Alm et al., 2015; Kazmierczak et al., The rapid global dissemination of Ambler class B metallo-β- 2016; Periasamy et al., 2020; Poirel et al., 2000; Watanabe et al., lactamase (MBL) enzymes along with their increasing diversity across 1991). -

Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and Its Application
Infect Dis Ther (2017) 6:57–67 DOI 10.1007/s40121-016-0144-8 REVIEW Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and its Application Juwon Yim . Leah M. Molloy . Jason G. Newland Received: November 10, 2016 / Published online: December 30, 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com ABSTRACT infections, CABP caused by penicillin- and ceftriaxone-resistant S. pneumoniae and Ceftaroline is a novel cephalosporin recently resistant Gram-positive infections that fail approved in children for treatment of acute first-line antimicrobial agents. However, bacterial skin and soft tissue infections and limited data are available on tolerability in community-acquired bacterial pneumonia neonates and infants younger than 2 months (CABP) caused by methicillin-resistant of age, and on pharmacokinetic characteristics Staphylococcus aureus, Streptococcus pneumoniae in children with chronic medical conditions and other susceptible bacteria. With a favorable and those with invasive, complicated tolerability profile and efficacy proven in infections. In this review, the microbiological pediatric patients and excellent in vitro profile of ceftaroline, its mechanism of action, activity against resistant Gram-positive and and pharmacokinetic profile will be presented. Gram-negative bacteria, ceftaroline may serve Additionally, clinical evidence for use in as a therapeutic option for polymicrobial pediatric patients and proposed place in therapy is discussed. Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/ 1F47F0601BB3F2DD. Keywords: Antibiotic resistance; Ceftaroline J. Yim (&) fosamil; Children; Methicillin-resistant St. John Hospital and Medical Center, Detroit, MI, Staphylococcus aureus; Streptococcus pneumoniae USA e-mail: [email protected] L. -

Global Assessment of the Antimicrobial Activity of Polymyxin B Against 54 731 Clinical Isolates of Gram-Negative Bacilli
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ORIGINAL ARTICLE 10.1111/j.1469-0691.2005.01351.x Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004) A. C. Gales1, R. N. Jones2,3 and H. S. Sader1,2 1Division of Infectious Diseases, Universidade Federal de Sa˜o Paulo, Sa˜o Paulo, Brazil, 2JMI Laboratories, North Liberty, IA, USA, and 3Tufts University School of Medicine, Boston, MA, USA ABSTRACT In total, 54 731 Gram-negative bacilli isolated worldwide between 2001 and 2004 from diverse sites of infection were tested for susceptibility to polymyxin B by the broth reference microdilution method, with interpretation of results according to CLSI (formerly NCCLS) guidelines. Polymyxin B showed excellent potency and spectrum against 8705 Pseudomonas aeruginosa and 2621 Acinetobacter spp. isolates (MIC50, £ ⁄ ⁄ 1mg L and MIC90,2mg L for both pathogens). Polymyxin B resistance rates were slightly higher for carbapenem-resistant P. aeruginosa (2.7%) and Acinetobacter spp. (2.8%), or multidrug-resistant (MDR) P. aeruginosa (3.3%) and Acinetobacter spp. (3.2%), when compared with the entire group (1.3% for P. aeruginosa and 2.1% for Acinetobacter spp.). Among P. aeruginosa, polymyxin B resistance rates varied from 2.9% in the Asia-Pacific region to only 1.1% in Europe, Latin America and North America, while polymyxin B resistance rates ranged from 2.7% in Europe to 1.7% in North America and Latin America £ ⁄ % among Acinetobacter spp. -

Synergistic Activity of Ampicillin and Cloxacillin
144 THE JOURNAL OF ANTIBIOTICS APR. 1969 SYNERGISTIC ACTIVITY OF AMPICILLIN AND CLOXACILLIN PROTECTIVE EFFECT OF CLOXACILLIN ON ENZYMATIC DEGRADATION OF AMPICILLIN BY PENICILLINASE, AND THERAPEUTIC ACTIVITY OF MIXTURES OF AMPICILLIN AND CLOXACILLIN MlNORUNlSHIDA, YASUHIROMlNE Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan and Shogo Kuwahara Department of Microbiology, Toho University School of Medicine, Tokyo, Japan (Received for publication February 15, 1969) A mixture of ampicillin (aminobenzyl penicillin, AB-PC) and cloxacillin (methylchlorophenylisoxazolyl penicillin, MCI-PC) showed an excellent bacteri- cidal activity even at low concentrations in which either of the penicillins alone did not have any activity against a clinically isolated strain of Escherichia colt, resistant to both of these penicillins. In this case, the microbial degrada- ion of AB-PC, caused by penicillinase (PC-ase), was strongly inhibited in the presence of MCI-PC. The enzymatic hydrolysis of AB-PC by extracellular nd cell-bound PC-ase, obtained from an AB-PC resistant strain of Staphylo- coccus aureus, was also inhibited in the presence of MCI-PC. Against that, t a under comparable conditions, penicillin G (PC-G) was almost completely destroyed by cell-bound PC-ase from this organism. An in vivo synergism was observed when mixtures of AB-PC and MCI-PC were applied for the treatment of mice challenged with a clinically isolated strain of E. coli resistant to both of these penicillins. Similar results were obtained in the treatment of mice experimentally infected with a strain of Staphylococcus aureus, which is highly resistant to AB-PC, but sensitive to MCI-PC. A decrease in the rate of hydrolysis of methicillin (dimethoxyphenyl penicillin, DMP-PC) by staphylococcal penicillinase (PC-ase) was first noted by Rolinson and his co-workers^. -

Cefalexin in the WHO Essential Medicines List for Children Reject
Reviewer No. 1: checklist for application of: Cefalexin In the WHO Essential Medicines List for Children (1) Have all important studies that you are aware of been included? Yes No 9 (2) Is there adequate evidence of efficacy for the proposed use? Yes 9 No (3) Is there evidence of efficacy in diverse settings and/or populations? Yes 9 No (4) Are there adverse effects of concern? Yes 9 No (5) Are there special requirements or training needed for safe/effective use? Yes No 9 (6) Is this product needed to meet the majority health needs of the population? Yes No 9 (7) Is the proposed dosage form registered by a stringent regulatory authority? Yes 9 No (8) What action do you propose for the Committee to take? Reject the application for inclusion of the following presentations of cefalexin: • Tablets/ capsules 250mg • Oral suspensions 125mg/5ml and 125mg/ml (9) Additional comment, if any. In order to identify any additional literature, the following broad and sensitive search was conducted using the PubMed Clinical Query application: (cephalexin OR cefalexin AND - 1 - pediatr*) AND ((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading]) Only one small additional study was identified, which looked at the provision of prophylactic antibiotics in patients presenting to an urban children's hospital with trauma to the distal fingertip, requiring repair.1 In a prospective randomised control trial, 146 patients were enrolled, of which 69 were randomised to the no-antibiotic group, and 66 were randomised to the antibiotic (cefalexin) group. -

Cefixime (Suprax) Division of Disease Control What Do I Need to Know?
Division of Disease Control What Do I Need To Know? Cefixime (Suprax) About your medicine: Cefixime is used to treat bacterial infections in many different parts of the body. This medicine will not work for colds, flu, or other viral infections. It may be used to other problems as determined by your healthcare provider. These instructions will help you receive the optimum benefits from Cefixime. Before taking this medication: Tell your doctor, nurse or healthcare provider if you… . Have any allergies to medications (especially cephalosporins and penicillin) . Take any other medications . Have medical condition (especially kidney or eye problems) . Are pregnant, breast-feeding or trying to become pregnant . History of convulsions Possible side effects of this medicine: If these symptoms continue or become severe, call your health care provider… . Nausea, vomiting, diarrhea . Gas . Indigestion . Dyspepsia (upset stomach) . Abdominal pain . Muscle cramps, stiffness, spasms . If your symptoms do not improve within a few days, notify your healthcare provider How to take this medicine: . This is the only dose of this medicine you will receive . Take this medication with a full glass (8 ounces) of water, preferably after a meal . DO NOT take with milk or other dairy products, or any products containing magnesium or calcium (such as antacids), iron or aluminum within 2 hours of the time you take this medicine . Drink several additional glasses of water today Warning: Call a healthcare professional immediately if you develop any of the following: . Difficulty breathing . Hives/skin rash Sexual Behavior: Abstain from any type of sexual activity for 7 days after treatment of self and partner For more information, contact the NDDoH STD Program at 701.328.2378 or toll-free 800.472.2180 Page 1 of 1 Last Updated: 4/3/2012 . -

Severe Sepsis and Septic Shock Antibiotic Guide
Stanford Health Issue Date: 05/2017 Stanford Antimicrobial Safety and Sustainability Program Severe Sepsis and Septic Shock Antibiotic Guide Table 1: Antibiotic selection options for healthcare associated and/or immunocompromised patients • Healthcare associated: intravenous therapy, wound care, or intravenous chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, attendance at a hospital or hemodialysis clinic within the prior 30 days • Immunocompromised: Receiving chemotherapy, known systemic cancer not in remission, ANC <500, severe cell-mediated immune deficiency Table 2: Antibiotic selection options for community acquired, immunocompetent patients Table 3: Antibiotic selection options for patients with simple sepsis, community acquired, immunocompetent patients requiring hospitalization. Risk Factors for Select Organisms P. aeruginosa MRSA Invasive Candidiasis VRE (and other resistant GNR) Community acquired: • Known colonization with MDROs • Central venous catheter • Liver transplant • Prior IV antibiotics within 90 day • Recent MRSA infection • Broad-spectrum antibiotics • Known colonization • Known colonization with MDROs • Known MRSA colonization • + 1 of the following risk factors: • Prolonged broad antibacterial • Skin & Skin Structure and/or IV access site: ♦ Parenteral nutrition therapy Hospital acquired: ♦ Purulence ♦ Dialysis • Prolonged profound • Prior IV antibiotics within 90 days ♦ Abscess -
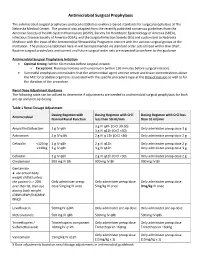
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Combination Therapy in Complicated Infections Due to S. Aureus
Combination therapy in complicated infections due to S. aureus Alex Soriano ([email protected]) Service of Infectious Diseases Hospital Clínic of Barcelona ESCMIDUniversity of Barcelona eLibrary IDIBAPS © by author Staphylococcal dissemination to different organs 30 min after i.v. Infection (using a real-time Sureward, et al. imaging) Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 ESCMID eLibrary © by author Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 ESCMID eLibrary Liver, Kupfer cell (purple ) © byS. aureus author(green) Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 eradication Large cluster of S. from blood aureus inside of KC. Located in phagolysosomes ESCMID eLibrary © by author Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 liver ESCMID eLibrary Vancosomes: vancomycin vancomycin (1h before ©or after by) authorwithin liposomes Surewaard, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016; 213: 1141-51 liver ESCMID eLibrary © by author Lehar S, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527: 1-19 ESCMID eLibrary © by author Lehar S, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527: 1-19 Animal model of S aureus bacteremia. Treatment started after 24h of the infection ESCMID eLibrary single dose © by author ESCMID CaseeLibrary #1 © by author Medical history: A 27 y-o man, without co-morbidity.