Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and Its Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cefditoren Pivoxil) Tablets 200 and 400 Mg
SPECTRACEF® (cefditoren pivoxil) Tablets 200 and 400 mg. To reduce the development of drug-resistant bacteria and maintain the effectiveness of SPECTRACEF® and other antibacterial drugs, SPECTRACEF® should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION SPECTRACEF® tablets contain cefditoren pivoxil, a semi-synthetic cephalosporin antibiotic for oral administration. It is a prodrug which is hydrolyzed by esterases during absorption, and the drug is distributed in the circulating blood as active cefditoren. Chemically, cefditoren pivoxil is (-)-(6R,7R)-2,2-dimethylpropionyloxymethyl 7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxy iminoacetamido]-3-[(Z)-2-(4-methylthiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate. The empirical formula is C25H28N6O7S3 and the molecular weight is 620.73. The structural formula of cefditoren pivoxil is shown below: cefditoren pivoxil The amorphous form of cefditoren pivoxil developed for clinical use is a light yellow powder. It is freely soluble in dilute hydrochloric acid and soluble at levels equal to 6.06 mg/mL in ethanol and <0.1 mg/mL in water. SPECTRACEF® (cefditoren pivoxil) tablets contain 200 mg or 400 mg of cefditoren as cefditoren pivoxil and the following inactive ingredients: croscarmellose sodium, D-mannitol, hydroxypropyl cellulose, hypromellose, magnesium stearate, sodium caseinate (a milk protein), and sodium tripolyphosphate. The tablet coating contains carnauba wax, hypromellose, polyethylene glycol, and titanium dioxide. Tablets are printed with ink containing D&C Red No. 27, FD&C Blue No. 1, propylene glycol, and shellac. CLINICAL PHARMACOLOGY Pharmacokinetics Absorption Oral Bioavailability Following oral administration, cefditoren pivoxil is absorbed from the gastrointestinal tract and hydrolyzed to cefditoren by esterases. -

P0469 In-Vitro Activity of Oritavancin Against Daptomycin And/Or Linezolid Resistant Enterococcus Faecium Clinical Isolates: a Single Centre Study
P0469 In-vitro activity of oritavancin against daptomycin and/or linezolid resistant Enterococcus faecium clinical isolates: a single centre study Abhay Dhand*1, Nicholas Feola1, Leslie Lee1, Guiqing Wang1 1Westchester Medical Center Background: Oritavancin is a novel antibiotic with potent activity against a broad range of gram- positive organisms. Resistance to daptomycin, linezolid and other antibiotics among enterococcus is on the rise in many US hospitals. Oritavancin may provide an alternative antibiotic therapy for serious infections caused by such very resistant enterococci. The aim of this study was to evaluate in vitro antimicrobial susceptibility of oritavancin against enterococci with focus on Daptomycin non- susceptible (DNSE) and Linezolid resistant (LRE) clinical isolates. Materials/methods: DNSE and LRE clinical isolates were recovered from patients in a tertiary medical center in suburban New York City from May 2015 through December 2016. Daptomycin susceptibility was determined by MicroScan WalkAway™ System and confirmed by E-test. Susceptibility of oritavancin and linezolid was performed by broth microdilution using the Sensititre™ panel in accordance with the guidelines of the Clinical and Laboratory Standards Institute and package insert of the manufacturer. The isolates were confirmed by analysis of 16S rRNA gene sequence or by the MALDI Biobiotyper CA System™. Results: A total of 24 nonduplicate E. faecium clinical isolates were analyzed in this study. 19/24 isolates were DNSE (6-96 μg/ml), 4/24 were LRE (8-16 μg/ml) and 2/24 were both DNSE and LRE. The MIC50 and MIC90 of oritavancin against combined resistant enterococci isolates was 0.06 μg/ml and 0.25 μg/ml (range: 0.008 – 0.25 μg/ml) respectively. -

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200327 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number(s) 200327 Priority or Standard Standard Submit Date(s) December 29, 2009 Received Date(s) December 30, 2009 PDUFA Goal Date October 30, 2010 Division / Office Division of Anti-Infective and Ophthalmology Products Office of Antimicrobial Products Reviewer Name(s) Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD Review Completion October 29, 2010 Date Established Name Ceftaroline fosamil for injection (Proposed) Trade Name Teflaro Therapeutic Class Cephalosporin; ß-lactams Applicant Cerexa, Inc. Forest Laboratories, Inc. Formulation(s) 400 mg/vial and 600 mg/vial Intravenous Dosing Regimen 600 mg every 12 hours by IV infusion Indication(s) Acute Bacterial Skin and Skin Structure Infection (ABSSSI); Community-acquired Bacterial Pneumonia (CABP) Intended Population(s) Adults ≥ 18 years of age Template Version: March 6, 2009 Reference ID: 2857265 Clinical Review Ariel Ramirez Porcalla, MD, MPH Neil Rellosa, MD NDA 200327: Teflaro (ceftaroline fosamil) Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ......................................... 9 1.1 Recommendation on Regulatory Action ........................................................... 10 1.2 Risk Benefit Assessment.................................................................................. 10 1.3 Recommendations for Postmarketing Risk Evaluation and Mitigation Strategies ........................................................................................................................ -

Ceftazidime for Injection) PHARMACY BULK PACKAGE – NOT for DIRECT INFUSION
PRESCRIBING INFORMATION FORTAZ® (ceftazidime for injection) PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION To reduce the development of drug-resistant bacteria and maintain the effectiveness of FORTAZ and other antibacterial drugs, FORTAZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Ceftazidime is a semisynthetic, broad-spectrum, beta-lactam antibacterial drug for parenteral administration. It is the pentahydrate of pyridinium, 1-[[7-[[(2-amino-4 thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1 azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, hydroxide, inner salt, [6R-[6α,7β(Z)]]. It has the following structure: The molecular formula is C22H32N6O12S2, representing a molecular weight of 636.6. FORTAZ is a sterile, dry-powdered mixture of ceftazidime pentahydrate and sodium carbonate. The sodium carbonate at a concentration of 118 mg/g of ceftazidime activity has been admixed to facilitate dissolution. The total sodium content of the mixture is approximately 54 mg (2.3 mEq)/g of ceftazidime activity. The Pharmacy Bulk Package vial contains 709 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3mEq) per gram of ceftazidime. FORTAZ in sterile crystalline form is supplied in Pharmacy Bulk Packages equivalent to 6g of anhydrous ceftazidime. The Pharmacy Bulk Package bottle is a container of sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous use. THE PHARMACY BULK PACKAGE IS NOT FOR DIRECT INFUSION, FURTHER DILUTION IS REQUIRED BEFORE USE. -

Empiric Antimicrobial Therapy for Diabetic Foot Infection
Empiric Antimicrobial Therapy for Diabetic Foot Infection (NB Provincial Health Authorities Anti-Infective Stewardship Committee, September 2019) Infection Severity Preferred Empiric Regimens Alternative Regimens Comments Mild Wound less than 4 weeks duration:d Wound less than 4 weeks duration:e • Outpatient management • Cellulitis less than 2 cm and • cephalexin 500 – 1000 mg PO q6h*,a OR • clindamycin 300 – 450 mg PO q6h (only if recommended ,a • cefadroxil 500 – 1000 mg PO q12h* severe delayed reaction to a beta-lactam) without involvement of deeper • Tailor regimen based on culture tissues and susceptibility results and True immediate allergy to a beta-lactam at MRSA Suspected: • Non-limb threatening patient response risk of cross reactivity with cephalexin or • doxycycline 200 mg PO for 1 dose then • No signs of sepsis cefadroxil: 100 mg PO q12h OR • cefuroxime 500 mg PO q8–12h*,b • sulfamethoxazole+trimethoprim 800+160 mg to 1600+320 mg PO q12h*,f Wound greater than 4 weeks duration:d Wound greater than 4 weeks duratione • amoxicillin+clavulanate 875/125 mg PO and MRSA suspected: q12h*,c OR • doxycycline 200 mg PO for 1 dose then • cefuroxime 500 mg PO q8–12h*,b AND 100 mg PO q12h AND metroNIDAZOLE metroNIDAZOLE 500 mg PO q12h 500 mg PO q12h OR • sulfamethoxazole+trimethoprim 800+160 mg to 1600/320 mg PO q12h*,f AND metroNIDAZOLE 500 mg PO q12h Moderate Wound less than 4 weeks duration:d Wound less than 4 weeks duration:e • Initial management with • Cellulitis greater than 2 cm or • ceFAZolin 2 g IV q8h*,b OR • levoFLOXacin 750 -

Anthem Blue Cross Drug Formulary
Erythromycin/Sulfisoxazole (generic) INTRODUCTION Penicillins ...................................................................... Anthem Blue Cross uses a formulary Amoxicillin (generic) (preferred list of drugs) to help your doctor Amoxicillin/Clavulanate (generic/Augmentin make prescribing decisions. This list of drugs chew/XR) is updated quarterly, by a committee Ampicillin (generic) consisting of doctors and pharmacists, so that Dicloxacillin (generic) the list includes drugs that are safe and Penicillin (generic) effective in the treatment of diseases. If you Quinolones ..................................................................... have any questions about the accessibility of Ciprofloxacin/XR (generic) your medication, please call the phone number Levofloxacin (Levaquin) listed on the back of your Anthem Blue Cross Sulfonamides ................................................................ member identification card. Erythromycin/Sulfisoxazole (generic) In most cases, if your physician has Sulfamethoxazole/Trimethoprim (generic) determined that it is medically necessary for Sulfisoxazole (generic) you to receive a brand name drug or a drug Tetracyclines .................................................................. that is not on our list, your physician may Doxycycline hyclate (generic) indicate “Dispense as Written” or “Do Not Minocycline (generic) Substitute” on your prescription to ensure Tetracycline (generic) access to the medication through our network ANTIFUNGAL AGENTS (ORAL) _________________ of community -

Cefalexin in the WHO Essential Medicines List for Children Reject
Reviewer No. 1: checklist for application of: Cefalexin In the WHO Essential Medicines List for Children (1) Have all important studies that you are aware of been included? Yes No 9 (2) Is there adequate evidence of efficacy for the proposed use? Yes 9 No (3) Is there evidence of efficacy in diverse settings and/or populations? Yes 9 No (4) Are there adverse effects of concern? Yes 9 No (5) Are there special requirements or training needed for safe/effective use? Yes No 9 (6) Is this product needed to meet the majority health needs of the population? Yes No 9 (7) Is the proposed dosage form registered by a stringent regulatory authority? Yes 9 No (8) What action do you propose for the Committee to take? Reject the application for inclusion of the following presentations of cefalexin: • Tablets/ capsules 250mg • Oral suspensions 125mg/5ml and 125mg/ml (9) Additional comment, if any. In order to identify any additional literature, the following broad and sensitive search was conducted using the PubMed Clinical Query application: (cephalexin OR cefalexin AND - 1 - pediatr*) AND ((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading]) Only one small additional study was identified, which looked at the provision of prophylactic antibiotics in patients presenting to an urban children's hospital with trauma to the distal fingertip, requiring repair.1 In a prospective randomised control trial, 146 patients were enrolled, of which 69 were randomised to the no-antibiotic group, and 66 were randomised to the antibiotic (cefalexin) group. -

(CUA) and Cefprozil (CZ) in Pharmaceutical Drugs by RP-HPLC
American Journal of www.biomedgrid.com Biomedical Science & Research ISSN: 2642-1747 --------------------------------------------------------------------------------------------------------------------------------- Research Article Copyright@ MA Alfeen New Development Method for Determination of Cefuroxime Axetil (CUA) and Cefprozil (CZ) in Pharmaceutical Drugs by RP-HPLC MA Alfeen1* and Y Yildiz2 1Department of Chemistry, Al-Baath University, Syria 2Science Department, Centenary University, USA *Corresponding author: MA Alfeen, Department of Chemistry, Faculty of Second Science, Al-Baath University, Homs, Syria. To Cite This Article: MA Alfeen.New Development Method for Determination of Cefuroxime Axetil (CUA) and Cefprozil (CZ) in Pharmaceutical Drugs by RP-HPLC. Am J Biomed Sci & Res. 2019 - 4(1). AJBSR.MS.ID.000759. DOI: 10.34297/AJBSR.2019.04.000759 Received: June 29, 2019 | Published: July 17, 2019 Abstract High performance liquid chromatography was one of the most important technologies used in drug control and pharmaceutical quality control. In this study, an analytical method was developed using chromatography method for determination of two Cephalosporin as like: Cefuroxime Axetil (CUA), and Cefprozil (CZ) in pharmaceutical Drug Formulations. Isocratic separation was performed on an Enable C18 column (125mm × 4.6mm i.d, 5.0μm, 10A˚) Using Triethylamine: Methanol: Acetonitrile: Ultra-Pure Water (0.1: 5: 25: 69.9 v/v/v/v %) as a mobile phase at flow rate of 1.5 mL\min. The PDA detection wavelength was set at 262nm. The linearity was observed over a concentration range of (0.01–50μg\mL) for RP-HPLC method (correlation coefficient=0.999). The developed method was validated according to ICH guidelines. The relative standard deviation values for the method precision studies were < 1%, and an accuracy was > 98%. -

Adverse Drug Reactions Sample Chapter
Sample copyright Pharmaceutical Press www.pharmpress.com 5 Drug-induced skin reactions Anne Lee and John Thomson Introduction Cutaneous drug eruptions are one of the most common types of adverse reaction to drug therapy, with an overall incidence rate of 2–3% in hos- pitalised patients.1–3 Almost any medicine can induce skin reactions, and certain drug classes, such as non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics and antiepileptics, have drug eruption rates approaching 1–5%.4 Although most drug-related skin eruptions are not serious, some are severe and potentially life-threatening. Serious reac- tions include angio-oedema, erythroderma, Stevens–Johnson syndrome and toxic epidermal necrolysis. Drug eruptions can also occur as part of a spectrum of multiorgan involvement, for example in drug-induced sys- temic lupus erythematosus (see Chapter 11). As with other types of drug reaction, the pathogenesis of these eruptions may be either immunological or non-immunological. Healthcare professionals should carefully evalu- ate all drug-associated rashes. It is important that skin reactions are identified and documented in the patient record so that their recurrence can be avoided. This chapter describes common, serious and distinctive cutaneous reactions (excluding contact dermatitis, which may be due to any external irritant, including drugs and excipients), with guidance on diagnosis and management. A cutaneous drug reaction should be suspected in any patient who develops a rash during a course of drug therapy. The reaction may be due to any medicine the patient is currently taking or has recently been exposed to, including prescribed and over-the-counter medicines, herbal or homoeopathic preparations, vaccines or contrast media. -

'Cephalosporin Allergy' Label Is Misleading
VOLUME 41 : NUMBER 2 : APRIL 2018 ARTICLE ‘Cephalosporin allergy’ label is misleading Carlo L Yuson SUMMARY Immunology registrar1 Constance H Katelaris Penicillins and cephalosporins can cause a similar spectrum of allergic reactions at a similar rate. Immunologist2 Cross-reactive allergy between penicillins and cephalosporins is rare, as is cross-reaction within William B Smith 1 the cephalosporin group. Patients should therefore not be labelled ‘cephalosporin-allergic’. Immunologist Cross-reactive allergy may occur between cephalosporins (and penicillins) which share similar 1 Clinical Immunology and side chains. Allergy Royal Adelaide Hospital Generally, a history of a penicillin allergy should not rule out the use of cephalosporins, and a 2 Immunology and Allergy history of a specific cephalosporin allergy should not rule out the use of other cephalosporins. Unit Campbelltown Hospital Specialist advice or further investigations may be required when the index reaction was New South Wales anaphylaxis or a severe cutaneous adverse reaction, or when the antibiotics in question share common side chains. Keywords When recording a drug allergy in the patient’s records, it is important to identify the specific drug cephalosporin allergy, hypersensitivity, penicillin suspected (or confirmed), along with the date and nature of the adverse reaction. Records need allergy to be updated after a negative drug challenge. Aust Prescr 2018;41:37–41 as Stevens-Johnson syndrome, toxic epidermal Introduction https://doi.org/10.18773/ necrolysis or acute generalised exanthematous To label an individual with a ‘cephalosporin allergy’ austprescr.2018.008 pustulosis or organ hypersensitivity). is misleading. Given the structural diversity of the cephalosporin family, hypersensitivity is seldom a Structural chemistry and allergy Corrected 3 December 2018 class effect but is much more likely to relate to the Immunological reactivity to small molecules such This is the corrected individual drug. -
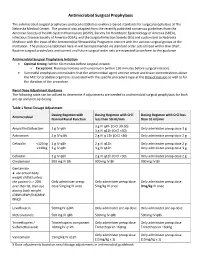
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Comparative Study of 5-Day and 10-Day Cefditoren Pivoxil Treatments for Recurrent Group a Β-Hemolytic Streptococcus Pharyngitis in Children
Hindawi Publishing Corporation International Journal of Pediatrics Volume 2009, Article ID 863608, 5 pages doi:10.1155/2009/863608 Clinical Study Comparative Study of 5-Day and 10-Day Cefditoren Pivoxil Treatments for Recurrent Group A β-Hemolytic Streptococcus pharyngitis in Children Hideaki Kikuta, Mutsuo Shibata, Shuji Nakata, Tatsuru Yamanaka, Hiroshi Sakata, Kouji Akizawa, and Kunihiko Kobayashi Pediatric Clinic, Touei Hospital, N-41, E-16, Higashi-ku, Sapporo 007-0841, Japan Correspondence should be addressed to Hideaki Kikuta, [email protected] Received 20 November 2008; Accepted 23 January 2009 Recommended by Samuel Menahem Efficacy of short-course therapy with cephalosporins for treatment of group A β-hemolytic streptococcus (GABHS) pharyngitis is still controversial. Subjects were 226 children with a history of at least one episode of GABHS pharyngitis. Recurrence within the follow-up period (3 weeks after initiation of therapy) occurred in 7 of the 77 children in the 5-day treatment group and in 1 of the 149 children in the 10-day treatment group; the incidence of recurrence being significantly higher in the 5-day treatment group. Bacteriologic treatment failure (GABHS isolation without overt pharyngitis) at follow-up culture was observed in 7 of the 77 children in the 5-day treatment group and 17 of the 149 children in the 10-day treatment group. There was no statistical difference between the two groups. A 5-day course of oral cephalosporins is not always recommended for treatment of GABHS pharyngitis in children who have repeated episodes of pharyngitis. Copyright © 2009 Hideaki Kikuta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.