Appendix 1—Reviewers and Contributers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Entomology Museum, University of Nebraska State 12-2009 Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii Mary Liz Jameson Wichita State University, [email protected] Darcy E. Oishi 2Hawaii Department of Agriculture, Plant Pest Control Branch, Honolulu, [email protected] Brett C. Ratcliffe University of Nebraska-Lincoln, [email protected] Grant T. McQuate USDA-ARS-PBARC, U.S. Pacific Basin Agricultural Research Center, Hilo, HI, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/entomologypapers Part of the Entomology Commons Jameson, Mary Liz; Oishi, Darcy E.; Ratcliffe, Brett C.; and McQuate, Grant T., "Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii" (2009). Papers in Entomology. 147. https://digitalcommons.unl.edu/entomologypapers/147 This Article is brought to you for free and open access by the Museum, University of Nebraska State at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. AProcddition. HawaiianAl inv AEsiventomol scA.r SAocbs. in(2009) HAwA 41:25–30ii 25 Two Additional Invasive Scarabaeoid Beetles (Coleoptera: Scarabaeidae: Dynastinae) in Hawaii Mary Liz Jameson1, Darcy E. Oishi2, Brett C. Ratcliffe3, and Grant T. McQuate4 1Wichita State University, Department of Biological Sciences, 537 Hubbard Hall, Wichita, Kansas 67260 [email protected]; 2Hawaii Department of Agriculture, Plant Pest Control Branch, 1428 South King St., Honolulu, HI 96814 [email protected]; 3University of Nebraska State Museum, Systematics Research Collections, W436 Nebraska Hall, University of Nebraska, Lincoln, Nebraska 68588 [email protected]; 4USDA-ARS-PBARC, U.S. -

Wooden and Bamboo Commodities Intended for Indoor and Outdoor Use
NAPPO Discussion Document DD 04: Wooden and Bamboo Commodities Intended for Indoor and Outdoor Use Prepared by members of the Pest Risk Analysis Panel of the North American Plant Protection Organization (NAPPO) December 2011 Contents Introduction ...........................................................................................................................3 Purpose ................................................................................................................................4 Scope ...................................................................................................................................4 1. Background ....................................................................................................................4 2. Description of the Commodity ........................................................................................6 3. Assessment of Pest Risks Associated with Wooden Articles Intended for Indoor and Outdoor Use ...................................................................................................................6 Probability of Entry of Pests into the NAPPO Region ...........................................................6 3.1 Probability of Pests Occurring in or on the Commodity at Origin ................................6 3.2 Survival during Transport .......................................................................................... 10 3.3 Probability of Pest Surviving Existing Pest Management Practices .......................... 10 3.4 Probability -
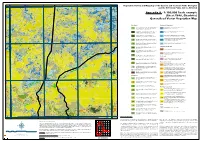
Appendix 9 - 1:100,000 Scale Example (Sheet 5648, Charlotte) Generalised Vector Vegetation Map
133°30'E 133°40'E 133°50'E 134°E 24°30'S Vegetation Survey and Mapping of the Eastern and Southern Finke Bioregion 24°30'S and the NT Stony Plains Inliers, NT & SA Appendix 9 - 1:100,000 Scale example (Sheet 5648, Charlotte) Generalised Vector Vegetation Map Woodland Chenopod Shrubland Acacia aneura ( Mulga) Low Open Woodland TO Tall Open Shrubland of Atriplex nummularia (Old man saltbush) Low Sparse Chenopod 1 Acacia estrophiolata (Ironwood) on clay loam plains and red earth 4 shrubland over Low Sparse Tussock grasses. soils+/- Atriplex vesicaria and Eragrostis eriopoda . Acacia georginae / Acacia cambagei ( Gidgee) Low Woodland to Tall Atriplex vesicaria (Pop saltbush) Low Open Chenopod Shrubland.+/- 2 Shrubland.+/- Eucalyptus coolabah subsp. Arida , Codonocarpus 5 Maireana astrotricha over tussock grasses. cotinifolius , Eulalia aurea, Eriachne ovata and Atriplex vesicaria . Eucalyptus coolabah subsp. arida (Coolabah) Woodland. +/- Maireana aphylla (Cottonbush) Low Sparse Chenopod Shrubland. +/- 12 Muehlenbeckia florulenta , Acacia aneura , Senna artemisioides ssp. 8 Fimbristylis dichotoma , Dactyloctenium radulans and Eragrostis dielsii. Filifolia , Marsilea sp ., Cynodon dactylon , and Cenchrus ciliaris . Maireana astrotricha (Low bluebush) Low Sparse Chenopod Shrubland Eucalyptus camaldulensis var. obtusa (River red gum) Woodland.+/- TO Sparse shrubland of Senna artemisioides n. coriacea and 13 Eucalyptus coolabah subsp. arida , Cynodon dactylon , Eulalia aurea and 9 Eremophila duttonii (Harlequin fuchsia bush). Cyperus gymnocaulos . 24°40'S Hakea leucoptera subsp. leucoptera (Needlewood) Open Woodland. +- Sclerolaena (mixed) Low Sparse Chenopod Shrubland.+/- Enneapogon 24°40'S 14 Eremophila sturtii , Senna artemisioides ssp. filifolia , Hakea leucoptera 15 avenaceus Aristida contorta , Sporobolus actinocladus . subsp. leucoptera and Triodia basedowii . Acacia calcicola (Northern Myall) Sparse Woodland +/- Eremophila Samphire Shrubland 23 duttonii , Acacia calcicola , Atriplex vesicaria , Maireana georgei and mixed short grasses. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

New Species of Aenetus from Sumatra, Indonesia (Lepidoptera
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomofauna Jahr/Year: 2018 Band/Volume: 0039 Autor(en)/Author(s): Grehan John R., Witt Thomas Josef, Ignatyev Nikolay N. Artikel/Article: New Species of Aenetus from Sumatra, Indonesia (Lepidoptera: Hepialidae) and a 5,000 km biogeographic disjunction 849-862 Entomofauna 39/239/1 HeftHeft 19:##: 849-862000-000 Ansfelden, 31.2. Januar August 2018 2018 New Species of Aenetus from Sumatra, Indonesia (Lepidoptera: Hepialidae) and a 5,000 km biogeographic disjunction John R. GREHAN, THOMAS J. WITT & NIKOLAI IGNATEV Abstract Discovery of Aenetus sumatraensis nov.sp. from northern Sumatra expands the distribution range of this genus well into southeastern Asia along the Greater and Lesser Sunda. The species belongs to the A. tegulatus clade distributed between northern Australia, New Gui- nea, islands west of New Guinea and the Lesser Sunda that are separated by over 5,000 km from A. sumatraensis nov.sp.. The Sumatran record either represents local differentiation from a formerly widespread ancestor or it is part a larger distribution of the A. tegulatus clade in the Greater Sunda that has not yet been discovered. The bursa copulatrix of the Australian species referred to as A. tegulatus is different from specimens examined in New Guinea and Ambon Islands (locality for the type) and is therefore referred to here as A. ther- mistes stat.rev. Zusammenfassung Die Entdeckung von Aenetus sumatraensis nov.sp. in Nord-Sumatra dehnt das Verbreitungs- gebiet dieser Gattung aus bis nach Südostasien entlang der Großen und Kleinen Sundainseln. -

Multi-Species Mating Disruption in Cranberries (Ericales: Ericaceae): Early Evidence Using a Flowable Emulsion
Journal of Insect Science (2017) 17(2): 54; 1–6 doi: 10.1093/jisesa/iex025 Research article Multi-Species Mating Disruption in Cranberries (Ericales: Ericaceae): Early Evidence Using a Flowable Emulsion Shawn A. Steffan,1,2,3 Elissa M. Chasen,1,2 Annie E. Deutsch,2,4 and Agenor Mafra-Neto5 1USDA-ARS Vegetable Crops Research Unit, 1630 Linden Drive, Madison, WI 53706 ([email protected]; elissa.chasen@ ars.usda.gov), 2Department of Entomology, University of Wisconsin, 1630 Linden Drive, Madison, WI 53706 (steffan@entomology. wisc.edu; [email protected]; [email protected]), 3Corresponding author, e-mail: ([email protected]), 4Door County University of Wisconsin-Extension, 421 Nebraska St., Sturgeon Bay, WI 54235 ([email protected]), and 5ISCA Technologies, Incorporated, 1230 W. Spring St., Riverside, CA 92507 ([email protected]) Subject Editor: Cesar Rodriguez-Saona Received 11 October 2016; Editorial decision 11 March 2017 Abstract Pheromone-based mating disruption has proven to be a powerful pest management tactic in many cropping systems. However, in the cranberry system, a viable mating disruption program does not yet exist. There are commercially available pheromones for several of the major pests of cranberries, including the cranberry fruit- worm, Acrobasis vaccinii Riley (Lepidoptera: Pyralidae) and blackheaded fireworm, Rhopobota naevana (Hu¨ bner) (Lepidoptera: Tortricidae). Previous studies have shown that mating disruption represents a promising approach for R. naevana management although carrier and delivery technologies have remained unresolved. The present study examined the suitability of Specialized Pheromone & Lure Application Technology (SPLAT; ISCA Technologies, Inc., Riverside, CA), a proprietary wax and oil blend, to serve as a pheromone carrier in the cranberry system. -

Study on the Consumption Index and Growth Rate of Acrotylus
Journal of Entomology and Zoology Studies 2016; 4(4): 407-412 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Study on the Consumption Index and Growth JEZS 2016; 4(7): 407-412 © 2016 JEZS Rate of Acrotylus humbertianus Saussure on Received: 17-05-2016 Accepted: 18-06-2016 Different Diet under Scanning Electron Muhammad Rafique Pitafi Microscope Department of Zoology, University of Sindh, Jamshoro, Pakistan Muhammad Rafique Pitafi, Riffat Sultana, Muhammad Saeed Wagan, Muhammad Kashif Sammon Riffat Sultana Department of Zoology, University of Sindh, Jamshoro, Abstract Pakistan Acrotylus humbertianus Saussure is a major agriculture pest in Sindh. This species consumes a wide variety of food plants from different families. In order to test it preference on different diets a conformity Muhammad Saeed Wagan chemical analysis has been done under Scanning Electron Microscope (SEM) for the first time. The Department of Zoology, maximum consumption index (CI) of all stages A. humbertianus on cabbage was recorded 0.042-0.78 University of Sindh, Jamshoro, mg/day followed by 0.2-0.42mg/d on sugarcane and 0.019-0.43mg/d on maize, while least CI was Pakistan calculated i-e 0.016-0.39 mg/day on mix diet. Beside this, growth rate (GR) of A. humbertianus on these food plants indicates that (GR) was highest on sugarcane i-e 0.016-0.25mg/d followed by 0.02 -0.62mg/d Muhammad Kashif Sammon and 0.013-0.35mg/d on cabbage and maize respectively, while it was 0.011-0.32 mg/d on mixed diet. The Center for Pure and Applied first and second instars of A. -

Highbush Blueberry: Cultivation, Protection, Breeding and Biotechnology
The European Journal of Plant Science and Biotechnology ©2007 Global Science Books Highbush Blueberry: Cultivation, Protection, Breeding and Biotechnology Daniele Prodorutti1* • Ilaria Pertot2 • Lara Giongo3 • Cesare Gessler2 1 Plant Protection Department, IASMA Research Centre, Via E. Mach 1, 38010 San Michele all’Adige (TN), Italy 2 Safecrop Centre, IASMA Research Centre, Via E. Mach 1, 38010 San Michele all’Adige (TN), Italy 3 Agrifood Quality Department, IASMA Research Centre, Via E. Mach 1, 38010 San Michele all’Adige (TN), Italy Corresponding author : * [email protected] ABSTRACT Highbush blueberry is one of the most commercially significant berry crops. It is mainly cultivated in the United States and Canada, but also in Europe, Australia, Chile and New Zealand. Production of this crop is likely to increase in response to increased consumer demand for healthy foods, including the antioxidant-rich blueberry. This review describes several issues and developments in sustainable blueberry farming, including agronomical and cultural techniques (mulching, irrigation, the beneficial effects of mycorrhizae and fertilization), disease management (biology and control of common and emerging diseases), pest management, pollinators (effects on fruit set and production), conventional breeding and molecular techniques for examining and engineering blueberry germplasm. This paper describes past problems and current challenges associated with the commercial production of highbush blueberry, as well as new approaches and techniques for -

Biological Inventory and Assessment Report, Fall 2018 Caltech Submillimeter Observatory, Maunakea, Hawai‘I
Biological Inventory and Assessment Report, Fall 2018 Caltech Submillimeter Observatory, Maunakea, Hawai‘i Action BoardApril 2019 Prepared for: Sustainable Resources Group Intn’l, Inc. Prepared by: Matthew J Medeiros, PhD [email protected] mattjmedeiros.comFor All photographs in this report are copyrighted by Matthew J Medeiros. TABLE OF CONTENTS 1 INTRODUCTION ................................................................................................................................ 1 1.1 Caltech Submillimeter Observatory Decommissioning ................................................................ 1 1.2 Physical Setting ............................................................................................................................. 1 2 METHODS ........................................................................................................................................... 3 2.1 Permit and Personnel .................................................................................................................... 3 2.2 Schedule ........................................................................................................................................ 3 2.3 Nomenclature ................................................................................................................................ 3 2.4 Methodology for Inventorying Plants, Lichens, Non-arthropod Animals, and Abiotic Features . 3 2.4.1 Transects: Floral and Abiotic Features ................................................................................ -

Volume 5 Pt 3
Conservation Science W. Aust. 7 (1) : 153–178 (2008) Flora and Vegetation of the banded iron formations of the Yilgarn Craton: the Weld Range ADRIENNE S MARKEY AND STEVEN J DILLON Science Division, Department of Environment and Conservation, Wildlife Research Centre, PO Box 51, Wanneroo WA 6946. Email: [email protected] ABSTRACT A survey of the flora and floristic communities of the Weld Range, in the Murchison region of Western Australia, was undertaken using classification and ordination analysis of quadrat data. A total of 239 taxa (species, subspecies and varieties) and five hybrids of vascular plants were collected and identified from within the survey area. Of these, 229 taxa were native and 10 species were introduced. Eight priority species were located in this survey, six of these being new records for the Weld Range. Although no species endemic to the Weld Range were located in this survey, new populations of three priority listed taxa were identified which represent significant range extensions for these taxa of conservation significance. Eight floristic community types (six types, two of these subdivided into two subtypes each) were identified and described for the Weld Range, with the primary division in the classification separating a dolerite-associated floristic community from those on banded iron formation. Floristic communities occurring on BIF were found to be associated with topographic relief, underlying geology and soil chemistry. There did not appear to be any restricted communities within the landform, but some communities may be geographically restricted to the Weld Range. Because these communities on the Weld Range are so closely associated with topography and substrate, they are vulnerable to impact from mineral exploration and open cast mining. -

The Jumping Plant-Lice (Hemiptera: Psylloidea) of the Maltese Islands
BULLETIN OF THE ENTOMOLOGICAL SOCIETY OF MALTA (2020) Vol. 11 : 103–117 DOI: 10.17387/BULLENTSOCMALTA.2020.18 The jumping plant-lice (Hemiptera: Psylloidea) of the Maltese Islands David MIFSUD* ABSTRACT. Twenty-one species of jumping plant-lice accommodated in five different families are here recorded from the Maltese Islands in an annotated checklist. The Aphalaridae is represented by four species (Agonoscena targionii (Lichtenstein), Blastopsylla occidentalis Taylor, Colposcenia aliena (Löw) and Glycaspis brimblecombei Moore), of which two (B. occidentalis and G. brimblecombei) are alien species originating from Australia. The Homotomidae is represented by Homotoma ficus (Linnaeus) and Macrohomotoma gladiata Kuwayama, the latter being an alien species originating from the Far East. The Liviidae is represented by Euphyllura olivina (Costa), Diaphorina lycii Loginova and Psyllopsis fraxinicola (Foerster). The Psyllidae is represented by Acizzia uncatoides (Ferris & Klyver), Cacopsylla myrthi (Puton) and C. pyri (Linnaeus), of which Acizzia uncatoides is an alien species originating from Australia. Finally, the most species-rich family is the Triozidae, represented by nine species (Bactericera albiventris (Foerster), B. crithmi (Löw), B. trigonica Hodkinson, Heterotrioza chenopodii (Reuter), Lauritrioza alacris (Flor), Trioza centranthi (Vallot), T. galii Foerster, T. kiefferi Giard and T. urticae (Linnaeus)). For each of the above species, collection data, distribution, host- plant data and other relevant information is provided. Lycium intricatum Boiss. is a new host-plant record for Diaphorina lycii, and Rhamnus lycioides subsp. oleoides (L.) Jahand. & Maire is a new host-plant record for Cacopsylla myrthi. A host- plant shift is documented for Bactericera crithmi, which alternates between Ferula melitensis Brullo et al. in winter and Crithmum maritimum L. -

Phytophthora Resistance and Susceptibility Stock List
Currently known status of the following plants to Phytophthora species - pathogenic water moulds from the Agricultural Pathology & Kingdom Protista. Biological Farming Service C ompiled by Dr Mary Cole, Agpath P/L. Agricultural Consultants since 1980 S=susceptible; MS=moderately susceptible; T= tolerant; MT=moderately tolerant; ?=no information available. Phytophthora status Life Form Botanical Name Family Common Name Susceptible (S) Tolerant (T) Unknown (UnK) Shrub Acacia brownii Mimosaceae Heath Wattle MS Tree Acacia dealbata Mimosaceae Silver Wattle T Shrub Acacia genistifolia Mimosaceae Spreading Wattle MS Tree Acacia implexa Mimosaceae Lightwood MT Tree Acacia leprosa Mimosaceae Cinnamon Wattle ? Tree Acacia mearnsii Mimosaceae Black Wattle MS Tree Acacia melanoxylon Mimosaceae Blackwood MT Tree Acacia mucronata Mimosaceae Narrow Leaf Wattle S Tree Acacia myrtifolia Mimosaceae Myrtle Wattle S Shrub Acacia myrtifolia Mimosaceae Myrtle Wattle S Tree Acacia obliquinervia Mimosaceae Mountain Hickory Wattle ? Shrub Acacia oxycedrus Mimosaceae Spike Wattle S Shrub Acacia paradoxa Mimosaceae Hedge Wattle MT Tree Acacia pycnantha Mimosaceae Golden Wattle S Shrub Acacia sophorae Mimosaceae Coast Wattle S Shrub Acacia stricta Mimosaceae Hop Wattle ? Shrubs Acacia suaveolens Mimosaceae Sweet Wattle S Tree Acacia ulicifolia Mimosaceae Juniper Wattle S Shrub Acacia verniciflua Mimosaceae Varnish wattle S Shrub Acacia verticillata Mimosaceae Prickly Moses ? Groundcover Acaena novae-zelandiae Rosaceae Bidgee-Widgee T Tree Allocasuarina littoralis Casuarinaceae Black Sheoke S Tree Allocasuarina paludosa Casuarinaceae Swamp Sheoke S Tree Allocasuarina verticillata Casuarinaceae Drooping Sheoak S Sedge Amperea xipchoclada Euphorbaceae Broom Spurge S Grass Amphibromus neesii Poaceae Swamp Wallaby Grass ? Shrub Aotus ericoides Papillionaceae Common Aotus S Groundcover Apium prostratum Apiaceae Sea Celery MS Herb Arthropodium milleflorum Asparagaceae Pale Vanilla Lily S? Herb Arthropodium strictum Asparagaceae Chocolate Lily S? Shrub Atriplex paludosa ssp.