HPAE-PAD Determination of Carbohydrates in Honey to Evaluate Samples for Quality and Adulteration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Author Version (PDF)
Green Chemistry Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/greenchem Page 1 of 30 Green Chemistry 1 A sustainable biotechnological process for the efficient synthesis of kojibiose 2 3 Marina Díez-Municio, Antonia Montilla, F. Javier Moreno * and Miguel Herrero 4 5 Instituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM), CEI 6 (UAM+CSIC), C/ Nicolás Cabrera 9, 28049 Madrid, Spain. 7 8 * Corresponding author: Tel.: +34 91 0017948; E-mail address: [email protected] 9 GreenChemistry Accepted Manuscript Green Chemistry Page 2 of 30 10 ABSTRACT 11 This work reports the optimization of a cost-effective and scalable process for 12 the enzymatic synthesis of kojibiose (2-O-α-D-glucopyranosyl-α-D-glucose) from 13 readily available and low-cost substrates such as sucrose and lactose. -

And Honeydew Sugars with Respect to Their Utilization by the Hymenopteran Parasitoid Cotesia Glomerata F.L
Journal of Insect Physiology 47 (2001) 1077–1084 www.elsevier.com/locate/jinsphys A comparison of nectar- and honeydew sugars with respect to their utilization by the hymenopteran parasitoid Cotesia glomerata F.L. Wa¨ckers * Institute of Plant Sciences, Applied Entomology, Swiss Federal Institute of Technology (ETH), 8092 Zurich, Switzerland Received 10 October 2000; received in revised form 12 February 2001; accepted 19 February 2001 Abstract Fourteen naturally occurring sugars were individually tested with respect to their effect on Cotesia glomerata longevity. Parasitoids kept with solutions of either sucrose, glucose and fructose lived for Ͼ30 days. This constitutes a factor 15 increase in life span in comparison to control individuals kept with water only. Stachyose, mannose, melezitose, melibiose, maltose and erlose increased parasitoid longevity by a factor of 11.2–6.9. Solutions of galactose and trehalose had a marginal, but still significant effect. Lactose and raffinose did not raise parasitoid longevity, while rhamnose actually reduced parasitoid survival. In an additional experiment, the relationship between quantity of sugar consumption and longevity was established for all 14 sugars. To study the effect of an unsuitable sugar in sugar mixtures, a range of glucose:rhamnose mixtures was tested. Even at 20% of the sugar mixture rhamnose suppressed the nutritional benefit of the 80% glucose. The nutritional suitability of the sugars shows a positive correlation with the previously reported gustatory response towards the individual sugars. Patterns of sugar utilization are discussed with respect to hydrolytic enzymes and carbohydrate biochemical characteristics. Our findings for C. glomerata are compared to patterns of sugar utilization reported for other species. -

Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information Poly(ionic liquid)s as a Distinct Receptor Material to Create Highly- Integrated Sensing Platform for Efficiently Identifying a Myriad of Saccharides Wanlin Zhang, Yao Li, Yun Liang, Ning Gao, Chengcheng Liu, Shiqiang Wang, Xianpeng Yin, and Guangtao Li* *Corresponding authors: Guangtao Li ([email protected]) S1 Contents 1. Experimental Section (Page S4-S6) Materials and Characterization (Page S4) Experimental Details (Page S4-S6) 2. Figures and Tables (Page S7-S40) Fig. S1 SEM image of silica colloidal crystal spheres and PIL inverse opal spheres. (Page S7) Fig. S2 Adsorption isotherm of PIL inverse opal. (Page S7) Fig. S3 Dynamic mechanical analysis and thermal gravimetric analysis of PIL materials. (Page S7) Fig. S4 Chemical structures of 23 saccharides. (Page S8) Fig. S5 The counteranion exchange of PIL photonic spheres from Br- to DCA. (Page S9) Fig. S6 Reflection and emission spectra of spheres for saccharides. (Page S9) Table S1 The jack-knifed classification on single-sphere array for 23 saccharides. (Page S10) Fig. S7 Lower detection concentration at 10 mM of the single-sphere array. (Page S11) Fig. S8 Lower detection concentration at 1 mM of the single-sphere array. (Page S12) Fig. S9 PIL sphere exhibiting great pH robustness within the biological pH range. (Page S12) Fig. S10 Exploring the tolerance of PIL spheres to different conditions. (Page S13) Fig. S11 Exploring the reusability of PIL spheres. (Page S14) Fig. S12 Responses of spheres to sugar alcohols. (Page S15) Fig. -

Determination of Carbohydrates in Honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA
Determination of carbohydrates in honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA ABSTRACT RESULTS SAMPLE ANALYSIS METHOD ACCURACY Table 7. Adulteration parameters for HS6 adulterated with 10% SS1 through SS5. Purpose: To develop an HPAE-PAD method for the determination of carbohydrates in honey Honey sugar analysis Sample Recovery HS6 (Wild Mountain Honey) samples to evaluate their quality and to assess the possibility of adulteration. Separation Adulteration Honey sugars were separated using a Dionex CarboPac PA210-Fast-4μm column (150 × 4 mm) in Method accuracy was evaluated by measuring recoveries of 10 sugar standards spiked into honey Parameters 100% + 10% + 10% + 10% + 10% + 10% For this study, we purchased 12 commercial honey samples (Table 1) and analyzed them using Honey SS1 SS2 SS3 SS4 SS5 Methods: Separation of individual honey sugars was achieved on the recently introduced Thermo series with a Dionex CarboPac PA210 guard column (50 × 4 mm). The column selectivity allow samples. For spiking experiments, four honey samples were used (HS7–HS10) and spiked with a 10- HPAE-PAD. Figure 3 shows the representative chromatograms of 3 honey samples. For all 12 Glucose(G), mg/L 121 115 116 117 119 107 Scientific™ Dionex™ CarboPac™ PA210-Fast-4μm column. Carbohydrate detection was by pulsed carbohydrates to be separated with only a hydroxide eluent generated using an eluent generator. A sugar standard mix at two concentration levels. Figure 4 shows the representative chromatograms investigated honey samples, fructose and glucose (Peak 2 and Peak 3), were found to be the major Fructose(F), mg/L 127 115 115 116 126 116 amperometric detection (PAD) with a gold working electrode and, therefore, no sample derivatization solution of honey sugar standards was prepared and an aliquot (10 μL) of the solution was injected of unspiked and spiked honey sample HS7. -

New Computational Approaches for Investigating the Impact of Mutations on the Transglucosylation Activity of Sucrose Phosphorylase Enzyme
Thesis submitted to the Université de la Réunion for award of Doctor of Philosophy in Sciences speciality in bioinformatics New computational approaches for investigating the impact of mutations on the transglucosylation activity of sucrose phosphorylase enzyme Mahesh VELUSAMY Thesis to be presented on 18th December 2018 in front of the jury composed of Mme Isabelle ANDRE, Directeur de Recherche CNRS, INSA de TOULOUSE, Rapporteur M. Manuel DAUCHEZ, Professeur, Université de Reims Champagne Ardennes, Rapporteur M. Richard DANIELLOU, Professeur, Université d'Orléans, Examinateur M. Yves-Henri SANEJOUAND, Directeur de Recherche CNRS, Université de Nantes, Examinateur Mme Irène MAFFUCCI, Maître de Conférences, Université de Technologie de Compiègne, Examinateur Mme Corinne MIRAL, Maître de Conférences HDR, Université de Nantes, Examinateur M. Frédéric CADET, Professeur, Université de La Réunion, Co-directeur de thèse M. Bernard OFFMANN, Professeur, Université de Nantes, Directeur de thèse ெ்்ந்ி ிைு்ூுத் ெ்யாம் ெ்த உதி்ு ையகு் ானகு் ஆ்ற் அிு. -ிு்ு் ுதி் அ்ப் ுுக், எனு த்ைத ேுாி, தா் க்ூி, அ்ண் ு்ு்ுமா், த்ைக பா்பா, ஆ்தா ுு்மா், ீனா, அ்ண் ்ுக், ெபிய்பா, ெபிய்மா ம்ு் உுுைணயா் இு்த அைண்ு ந்ப்கு்ு் எனு மனமா்்த ந்ி. இ்த ஆ்ி்ைக ுுைம அை3த்ு ுுுத்்காரண், எனு ஆ்ி்ைக இய்ுன் ேபராிிய் ெப்னா்் ஆஃ்ேம். ப்ேு துண்கி் நா் மனதாு், ெபாுளாதார அளிு் க்3்ி் இு்தேபாு, என்ு இ்ெனாு த்ைதயாகே இு்ு எ்ைன பா்்ு்ெகா்3ா். ுி்பாக, எனு ூ்றா் ஆ்ு இுிி், அ்்ு எ்ளோ தி்ப்3 க3ைமக் ம்ு் ிர்ிைனக் இு்தாு், அைத்ெபாு்பு்தாு, அ் என்ு ெ்த ெபாுளாதார உதி, ப்கைB்கழக பிு ம்ு் இதர ி்ாக ்ப்த்ப்3 உதிகு்ு எ்ன ைகமா்ு ெகாு்தாு் ஈ3ாகாு. -

Selective Fermentation of Potential Prebiotic Lactose-Derived Oligosaccharides By
1 Selective fermentation of potential prebiotic lactose-derived oligosaccharides by 2 probiotic bacteria 3 4 Tomás García-Cayuela, Marina Díez-Municio, Miguel Herrero, M. Carmen Martínez- 5 Cuesta, Carmen Peláez, Teresa Requena*, F. Javier Moreno 6 7 Instituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM), CEI 8 (UAM+CSIC), Nicolás Cabrera 9, 28049 Madrid, Spain. 9 10 * Corresponding author: Tel.: +34 91 0017900; E-mail address: [email protected] 11 1 12 Abstract 13 The growth of potential probiotic strains from the genera Lactobacillus, 14 Bifidobacterium and Streptococcus was evaluated with the novel lactose-derived 15 trisaccharides 4’-galactosyl-kojibiose and lactulosucrose and the potential prebiotics 16 lactosucrose and kojibiose. The novel oligosaccharides were synthesized from 17 equimolar sucrose:lactose and sucrose:lactulose mixtures, respectively, by the use of a 18 Leuconostoc mesenteroides dextransucrase and purified by liquid chromatography. The 19 growth of the strains using the purified carbohydrates as the sole carbon source was 20 evaluated by recording the culture optical density and calculating maximum growth 21 rates and lag phase parameters. The results revealed an apparent bifidogenic effect of 22 lactulosucrose, being also a moderate substrate for streptococci and poorlybut badly 23 utilized by lactobacilli. In addition, 4’-galactosyl-kojibiose was selectively fermented by 24 Bifidobacterium breve, which was also the only tested bifidobacterial species able to 25 ferment kojibiose. The described fermentation properties of the specific probiotic strains 26 on the lactose-derived oligosaccharides would enable the design of prebiotics with a 27 high degree of selectivity. 28 29 Keywords: Prebiotic; Lactose-derived oligosaccharides; Probiotic; Bifidobacterium; 30 Lactobacillus; Streptococcus 31 2 32 1. -

United States Patent (10) Patent No.: US 7,223,570 B2 Aga Et Al
US007223570B2 (12) United States Patent (10) Patent No.: US 7,223,570 B2 Aga et al. (45) Date of Patent: May 29, 2007 (54) BRANCHED CYCLIC TETRASACCHARIDE, 5,763,598 A 6/1998 Hamayasu et al. PROCESS FOR PRODUCING THE SAME, 5,786, 196 A * 7/1998 Cote et al. .................. 435,208 AND USE FOREIGN PATENT DOCUMENTS (75) Inventors: Hajime Aga, Okayama (JP); Takanobu f Higashiyama, Okayama (JP); Hikaru E. 1 . A1 is: Watanabe, Okayama (JP); Tomohiko JP 647s) 1, 1994 Sonoda, Okayama (JP); Michio JP 6-16705 1, 1994 Kubota, Okayama (JP) JP 6-298806 10, 1994 JP 10-25305 1, 1998 (73) Assignee: Kabushiki Kaisha Hayashibara JP 10-304882 11, 1998 Seibutsu Kagaku Kenkyujo, Okayama WO WO 01/90338 A1 11, 2001 (JP) WO WO O2/10361 A1 2/2002 WO WO O2.40659 A1 5, 2002 (*) Notice: Subject to any disclaimer, the term of this WO WO O2/O55708 A1 T 2002 patent is extended or adjusted under 35 U.S.C. 154(b) by 324 days. OTHER PUBLICATIONS (21) Appl. No.: 10/471.377 Biely, P. “Enzymic alpha-galactosylation . " Carbohyd. Res. (2001) vol. 332, pp. 299-303.* (22) PCT Filed: Mar. 8, 2002 Cote, Gregory and Biely, Peter, Enzymically produced cyclic C-1,3- linked and O-1,6-linked oligosaccharides of D-glucose, European (86). PCT No.: PCT/UPO2/O2213 Journal of Biochemistry, vol. 226, (1994), p. 641-648. Sambrook, Joseph and Russell, David, Molecular Cloning. A Labo S 371 (c)(1), ratory Manuel. 3" Edition, (Cold Spring Harbor Laboratory, New (2), (4) Date: Sep. -

Ep 3117827 A1
(19) TZZ¥__ _T (11) EP 3 117 827 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 18.01.2017 Bulletin 2017/03 A61K 31/7016 (2006.01) A61P 15/02 (2006.01) (21) Application number: 15760638.5 (86) International application number: PCT/CN2015/073978 (22) Date of filing: 11.03.2015 (87) International publication number: WO 2015/135470 (17.09.2015 Gazette 2015/37) (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • ZENG, Zhongming GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Shenzhen, Guangdong 518000 (CN) PL PT RO RS SE SI SK SM TR • ZHOU, Ruyun Designated Extension States: Shenzhen, Guangdong 518000 (CN) BA ME Designated Validation States: (74) Representative: Krauns, Christian MA Wallinger Ricker Schlotter Tostmann Patent- und Rechtsanwälte Partnerschaft mbB (30) Priority: 13.03.2014 CN 201410103662 Zweibrückenstraße 5-7 80331 München (DE) (71) Applicant: SINGAPORE ZE&Z INTERNATIONAL PTE. LTD. Singapore 079903 (SG) (54) COMPOSITION FOR VAGINA AND USE OF THE COMPOSITION (57) A use of isomaltulose in the preparation of a vag- non-therapeutic vaginal care method, a non-therapeutic inal composition, wherein the amount of isomaltulose vaginal cleaning method, a method for improving vaginal used is 0.05-20% (w/w). A vaginal composition contains acidity, a method for promoting growth of Lactobacillus 0.05-20% (w/w) of isomaltulose. The composition may in the vagina, and a method for preventing and treating be a health product, health care product, cleaning prod- vaginal dysbacteriosis, especially bacterial vaginal dis- uct, skin care product, deodorant, cosmetic, or disinfect- eases, these methods being implemented by adminis- ant composition or a pharmaceutical composition. -
Supplementary Tables Vfinal.Xlsx
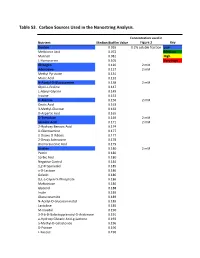
Table S3. Carbon Sources Used in the Nanostring Analysis. Concentration used in Nutrient Median Biofilm Value Figure 3 Key Dextrin 0.065 0.2% soluble fraction Low Melibionic Acid 0.072 Medium Mannan 0.081 High L-Homoserine 0.105 Very High Glycogen 0.120 2 mM Adenosine 0.127 2 mM Methyl Pyruvate 0.131 Mucic Acid 0.132 N-Acetyl-D-Glucosamine 0.138 2 mM Glycil-L-Proline 0.147 L-Alanyl-Glycine 0.149 Inosine 0.153 D-Alanine 0.156 2 mM Oxalic Acid 0.163 3-Methyl-Glucose 0.163 D-Aspartic Acid 0.165 D-Trehalose 0.169 2 mM Glycolic Acid 0.171 2 mM 2-Hydroxy Benzoic Acid 0.174 D-Glucosamine 0.177 2-Deoxy-D-Ribose 0.177 2-Deoxy Adenosine 0.178 Bromo Succinic Acid 0.179 Uridine 0.180 2 mM Pectin 0.180 Sorbic Acid 0.180 Negative Control 0.184 1,2-Propanediol 0.185 a-D-Lactose 0.186 Gelatin 0.186 D,L-a-Glycerol-Phosphate 0.186 Maltotriose 0.186 Glycerol 0.188 Inulin 0.189 Glucuronamide 0.189 N-Acetyl-D-Glucosaminatol 0.189 Lactulose 0.189 M-Inositol 0.190 3-0-b-D-Galactopyranosyl-D-Arabinose 0.191 a-Hydroxy Glutaric Acid-g-Lactone 0.193 a-Methyl-D-Galactoside 0.196 D-Psicose 0.196 L-Fucose 0.196 Succinamic Acid 0.197 D-Sorbitol 0.197 2 mM Dulcitol 0.198 D-Fructose-6-Phosphate 0.198 Sedoheptulosa 0.199 Glyoxylic Acid 0.200 Maltose 0.201 2 mM D-Ribono-1,4-Lactone 0.201 Sucrose 0.201 2 mM D-Melibiose 0.202 Mono Methyl Succinate 0.203 N-Acetyl-b-D-Mannosamine 0.204 p-Hydroxy Phenyl Acetic Acid 0.204 L-Rhamnose 0.205 2 mM D-Cellobiose 0.206 D-Glucuronic Acid 0.207 Adonitol 0.207 b-Methyl-D-Glucoside 0.207 Laminarin 0.208 Acetoacetic Acid 0.208 a-Keto-Glutaric -

Carbohydrate, Oligosaccharide, and Organic Acid Separations
Carbohydrate, Oligosaccharide, and Organic Acid Separations Long column lifetimes. Accurate, reproducible analysis. Trust Rezex™ For Excellent Resolution and Reproducibility of Sugars, Starches, and Organic Acids Phenomenex Rezex HPLC ion-exclusion columns are guaranteed to give you the performance you need. From drug formulation and excipient analysis to quality control testing of finished food products, Rezex columns consistently provide accurate and reproducible results. “ I have been using Phenomenex Rezex …for sugar quantitation in the last few years, we are very happy with the resolution of this column, plus a short running time. ” - Global Leader, Food & Beverage Ingredient Production Try Rezex Risk Free! Rezex is a guaranteed alternative to: • Bio-Rad® Aminex® If you are not completely satisfied with the performance Waters® Sugar-Pak™ • of any Rezex column, as compared to a competing Supelco® SUPELCOGEL™ product of the same size and phase, simply return the • Rezex column with your comparative data within 45 • Transgenomic® CARBOSep™ days for a FULL REFUND. Experience the Rezex™ Performance Advantage Broad Range of Phases to Perfectly Suit Your Application Needs .........................................2 Column Selection – Variety Gives You the Power of Optimization .................................................3 See the Difference! The Rezex Performance Advantage Sharper Peak Shape = Easy & Accurate Quantitation .........................................................4 Lower Backpressure = Longer Column Lifetimes & Faster -

Grasnotice 946, Lactobacillus Plantarum Strain DSM 33452
GRAS Notice (GRN) No. 946 https://www.fda.gov/food/generally-recognized-safe-gras/ RECEIVED gras-notice-inventory JUNO 2 1020 Chr. Hansen, In -?FFICE OF FOOD ADD ITIVE SAFETY 9015 West Maple Street Milwaukee, WI 53214 - 4298 Division of Biotechnology and GRAS Notice Review U.S .A. Center for Food Safety & Applied Nutrition (HFS-255) U.S. Food & Drug Administration Phone 414 - 607 - 5700 Fax 414 - 607 - 5959 Reference: Lactobacillus plantarum DSM 33452 June 1, 2020 Dear Sir or Madam, In accordance with the Federal Register [81 Fed. Reg. 159 (17 August 2016)] issuance on Generally Recognized as Safe (GRAS) notifications (21 CFR Part 170), Chr. Hansen is pleased to submit a notice that we have concluded, through scientific procedures that Lactobacillus plantarum (L. plantarum) DSM 33452 is generally recognized as safe for use in malolactic fermentation of wine and musts and is not subject to the pre-market approval requirements. The recommendation is to inoculate the pure starter culture of L. plantarum DSM 33452 into wine or must at an inoculation level of 1.0E+07 CFU/g at the time of crushing grapes or as early as possible in the fermentation tanks. L. plantarum DSM 33452 is sensitive to alcohol concentration, so the concentration of the organism will decrease as the concentration of alcohol in the wine or must increases. Though L. plantarum DSM 33452 is safe to consume, it would be present at negligible levels, if at all, in the finished product. It should also be noted that due to recent taxonomic changes to the genus Lactobaci/lus, Lactobacillus plantarum will be known as Lactiplantibacillus plantarum moving forward (Zheng, et al., 2020). -

(Mangifera Indica) Starch
fluids Article Rheological and Microstructural Properties of Oil-in-Water Emulsion Gels Containing Natural Plant Extracts Stabilized with Carboxymethyl Cellulose/Mango (Mangifera indica) Starch Luis Mieles-Gómez 1 , Santander E. Lastra-Ripoll 1 , Edilbert Torregroza-Fuentes 2, Somaris E. Quintana 1 and Luis A. García-Zapateiro 1,* 1 Research Group of Complex Fluid Engineering and Food Rheology, University of Cartagena, Cartagena 130015, Colombia; [email protected] (L.M.-G.); [email protected] (S.E.L.-R.); [email protected] (S.E.Q.) 2 Research Group Science Technology and Society—CTS, University of Cartagena, Cartagena 130015, Colombia; [email protected] * Correspondence: [email protected]; Tel.:+57-5-675-20-24 (ext. 205) Abstract: Emulsion gels are an alternative to developing food products and adding bioactive com- pounds; however, different stabilizers have been employed considering natural ingredients. In this work, stabilization of emulsion gels with blends of carboxymethylcellulose and kernel mango starch was performed with the addition of mango peel extracts, evaluating their physical, rheological and microstructural properties. Phenolic extract from mango peels (yields = 11.35 ± 2.05% w/w), with Citation: Mieles-Gómez, L.; 294.60 ± 0.03 mg GAE/100 g of extract and 436.77 ± 5.30 µMol Trolox/g of the extract, was obtained Lastra-Ripoll, S.E.; by ultrasound-assisted extraction (1:10 peel: Ethanol w/v, 200 W, 30 min), containing pyrogallol, Torregroza-Fuentes, E.; Quintana, melezitose, succinic acid, γ-tocopherol, campesterol, stigmasterol, lupeol, vitamin A and vitamin E. S.E.; García-Zapateiro, L.A. In addition, mango kernel starch (yields = 59.51 ± 1.35% w/w) with 27.28 ± 0.05% of amylose was Rheological and Microstructural Mangifera indica Properties of Oil-in-Water Emulsion obtained, being the by-product of mango ( var fachir) an alternative to producing Gels Containing Natural Plant natural food ingredients.