Ep 3117827 A1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Honeydew Sugars with Respect to Their Utilization by the Hymenopteran Parasitoid Cotesia Glomerata F.L
Journal of Insect Physiology 47 (2001) 1077–1084 www.elsevier.com/locate/jinsphys A comparison of nectar- and honeydew sugars with respect to their utilization by the hymenopteran parasitoid Cotesia glomerata F.L. Wa¨ckers * Institute of Plant Sciences, Applied Entomology, Swiss Federal Institute of Technology (ETH), 8092 Zurich, Switzerland Received 10 October 2000; received in revised form 12 February 2001; accepted 19 February 2001 Abstract Fourteen naturally occurring sugars were individually tested with respect to their effect on Cotesia glomerata longevity. Parasitoids kept with solutions of either sucrose, glucose and fructose lived for Ͼ30 days. This constitutes a factor 15 increase in life span in comparison to control individuals kept with water only. Stachyose, mannose, melezitose, melibiose, maltose and erlose increased parasitoid longevity by a factor of 11.2–6.9. Solutions of galactose and trehalose had a marginal, but still significant effect. Lactose and raffinose did not raise parasitoid longevity, while rhamnose actually reduced parasitoid survival. In an additional experiment, the relationship between quantity of sugar consumption and longevity was established for all 14 sugars. To study the effect of an unsuitable sugar in sugar mixtures, a range of glucose:rhamnose mixtures was tested. Even at 20% of the sugar mixture rhamnose suppressed the nutritional benefit of the 80% glucose. The nutritional suitability of the sugars shows a positive correlation with the previously reported gustatory response towards the individual sugars. Patterns of sugar utilization are discussed with respect to hydrolytic enzymes and carbohydrate biochemical characteristics. Our findings for C. glomerata are compared to patterns of sugar utilization reported for other species. -

Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information Poly(ionic liquid)s as a Distinct Receptor Material to Create Highly- Integrated Sensing Platform for Efficiently Identifying a Myriad of Saccharides Wanlin Zhang, Yao Li, Yun Liang, Ning Gao, Chengcheng Liu, Shiqiang Wang, Xianpeng Yin, and Guangtao Li* *Corresponding authors: Guangtao Li ([email protected]) S1 Contents 1. Experimental Section (Page S4-S6) Materials and Characterization (Page S4) Experimental Details (Page S4-S6) 2. Figures and Tables (Page S7-S40) Fig. S1 SEM image of silica colloidal crystal spheres and PIL inverse opal spheres. (Page S7) Fig. S2 Adsorption isotherm of PIL inverse opal. (Page S7) Fig. S3 Dynamic mechanical analysis and thermal gravimetric analysis of PIL materials. (Page S7) Fig. S4 Chemical structures of 23 saccharides. (Page S8) Fig. S5 The counteranion exchange of PIL photonic spheres from Br- to DCA. (Page S9) Fig. S6 Reflection and emission spectra of spheres for saccharides. (Page S9) Table S1 The jack-knifed classification on single-sphere array for 23 saccharides. (Page S10) Fig. S7 Lower detection concentration at 10 mM of the single-sphere array. (Page S11) Fig. S8 Lower detection concentration at 1 mM of the single-sphere array. (Page S12) Fig. S9 PIL sphere exhibiting great pH robustness within the biological pH range. (Page S12) Fig. S10 Exploring the tolerance of PIL spheres to different conditions. (Page S13) Fig. S11 Exploring the reusability of PIL spheres. (Page S14) Fig. S12 Responses of spheres to sugar alcohols. (Page S15) Fig. -

Improving the Utilization of Isomaltose and Panose by Lager Yeast Saccharomyces Pastorianus
fermentation Article Improving the Utilization of Isomaltose and Panose by Lager Yeast Saccharomyces pastorianus Javier Porcayo Loza 1,2,† , Anna Chailyan 3, Jochen Forster 3 , Michael Katz 3, Uffe Hasbro Mortensen 2,* and Rosa Garcia Sanchez 3,* 1 Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark, 2800 Kongens Lyngby, Denmark; [email protected] 2 Department of Bioengineering, Technical University of Denmark, 2800 Kongens Lyngby, Denmark 3 Carlsberg A/S, Carlsberg Research Laboratory, 1799 Copenhagen V, Denmark; [email protected] (A.C.); [email protected] (J.F.); [email protected] (M.K.) * Correspondence: [email protected] (U.H.M.); [email protected] (R.G.S.) † Current address: Graphenea S.A., 20009 San Sebastian, Spain. Abstract: Approximately 25% of all carbohydrates in industrial worts are poorly, if at all, fermented by brewing yeast. This includes dextrins, β-glucans, arabinose, xylose, disaccharides such as isomaltose, nigerose, kojibiose, and trisaccharides such as panose and isopanose. As the efficient utilization of carbohydrates during the wort’s fermentation impacts the alcohol yield and the organoleptic traits of the product, developing brewing strains with enhanced abilities to ferment subsets of these sugars is highly desirable. In this study, we developed Saccharomyces pastorianus laboratory yeast strains with a superior capacity to grow on isomaltose and panose. First, we designed a plasmid toolbox for Citation: Porcayo Loza, J.; Chailyan, the stable integration of genes into lager strains. Next, we used the toolbox to elevate the levels of A.; Forster, J.; Katz, M.; Mortensen, the α-glucoside transporter Agt1 and the major isomaltase Ima1. -

Congenital Sucrase-Isomaltase Deficiency
Congenital sucrase-isomaltase deficiency Description Congenital sucrase-isomaltase deficiency is a disorder that affects a person's ability to digest certain sugars. People with this condition cannot break down the sugars sucrose and maltose. Sucrose (a sugar found in fruits, and also known as table sugar) and maltose (the sugar found in grains) are called disaccharides because they are made of two simple sugars. Disaccharides are broken down into simple sugars during digestion. Sucrose is broken down into glucose and another simple sugar called fructose, and maltose is broken down into two glucose molecules. People with congenital sucrase- isomaltase deficiency cannot break down the sugars sucrose and maltose, and other compounds made from these sugar molecules (carbohydrates). Congenital sucrase-isomaltase deficiency usually becomes apparent after an infant is weaned and starts to consume fruits, juices, and grains. After ingestion of sucrose or maltose, an affected child will typically experience stomach cramps, bloating, excess gas production, and diarrhea. These digestive problems can lead to failure to gain weight and grow at the expected rate (failure to thrive) and malnutrition. Most affected children are better able to tolerate sucrose and maltose as they get older. Frequency The prevalence of congenital sucrase-isomaltase deficiency is estimated to be 1 in 5, 000 people of European descent. This condition is much more prevalent in the native populations of Greenland, Alaska, and Canada, where as many as 1 in 20 people may be affected. Causes Mutations in the SI gene cause congenital sucrase-isomaltase deficiency. The SI gene provides instructions for producing the enzyme sucrase-isomaltase. -

Structural Features
1 Structural features As defined by the International Union of Pure and Applied Chemistry gly- cans are structures of multiple monosaccharides linked through glycosidic bonds. The terms sugar and saccharide are synonyms, depending on your preference for Arabic (“sukkar”) or Greek (“sakkēaron”). Saccharide is the root for monosaccha- rides (a single carbohydrate unit), oligosaccharides (3 to 20 units) and polysac- charides (large polymers of more than 20 units). Carbohydrates follow the basic formula (CH2O)N>2. Glycolaldehyde (CH2O)2 would be the simplest member of the family if molecules of two C-atoms were not excluded from the biochemical repertoire. Glycolaldehyde has been found in space in cosmic dust surrounding star-forming regions of the Milky Way galaxy. Glycolaldehyde is a precursor of several organic molecules. For example, reaction of glycolaldehyde with propenal, another interstellar molecule, yields ribose, a carbohydrate that is also the backbone of nucleic acids. Figure 1 – The Rho Ophiuchi star-forming region is shown in infrared light as captured by NASA’s Wide-field Infrared Explorer. Glycolaldehyde was identified in the gas surrounding the star-forming region IRAS 16293-2422, which is is the red object in the centre of the marked square. This star-forming region is 26’000 light-years away from Earth. Glycolaldehyde can react with propenal to form ribose. Image source: www.eso.org/public/images/eso1234a/ Beginning the count at three carbon atoms, glyceraldehyde and dihydroxy- acetone share the common chemical formula (CH2O)3 and represent the smallest carbohydrates. As their names imply, glyceraldehyde has an aldehyde group (at C1) and dihydoxyacetone a carbonyl group (at C2). -

Determination of Carbohydrates in Honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA
Determination of carbohydrates in honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA ABSTRACT RESULTS SAMPLE ANALYSIS METHOD ACCURACY Table 7. Adulteration parameters for HS6 adulterated with 10% SS1 through SS5. Purpose: To develop an HPAE-PAD method for the determination of carbohydrates in honey Honey sugar analysis Sample Recovery HS6 (Wild Mountain Honey) samples to evaluate their quality and to assess the possibility of adulteration. Separation Adulteration Honey sugars were separated using a Dionex CarboPac PA210-Fast-4μm column (150 × 4 mm) in Method accuracy was evaluated by measuring recoveries of 10 sugar standards spiked into honey Parameters 100% + 10% + 10% + 10% + 10% + 10% For this study, we purchased 12 commercial honey samples (Table 1) and analyzed them using Honey SS1 SS2 SS3 SS4 SS5 Methods: Separation of individual honey sugars was achieved on the recently introduced Thermo series with a Dionex CarboPac PA210 guard column (50 × 4 mm). The column selectivity allow samples. For spiking experiments, four honey samples were used (HS7–HS10) and spiked with a 10- HPAE-PAD. Figure 3 shows the representative chromatograms of 3 honey samples. For all 12 Glucose(G), mg/L 121 115 116 117 119 107 Scientific™ Dionex™ CarboPac™ PA210-Fast-4μm column. Carbohydrate detection was by pulsed carbohydrates to be separated with only a hydroxide eluent generated using an eluent generator. A sugar standard mix at two concentration levels. Figure 4 shows the representative chromatograms investigated honey samples, fructose and glucose (Peak 2 and Peak 3), were found to be the major Fructose(F), mg/L 127 115 115 116 126 116 amperometric detection (PAD) with a gold working electrode and, therefore, no sample derivatization solution of honey sugar standards was prepared and an aliquot (10 μL) of the solution was injected of unspiked and spiked honey sample HS7. -

Ii- Carbohydrates of Biological Importance
Carbohydrates of Biological Importance 9 II- CARBOHYDRATES OF BIOLOGICAL IMPORTANCE ILOs: By the end of the course, the student should be able to: 1. Define carbohydrates and list their classification. 2. Recognize the structure and functions of monosaccharides. 3. Identify the various chemical and physical properties that distinguish monosaccharides. 4. List the important monosaccharides and their derivatives and point out their importance. 5. List the important disaccharides, recognize their structure and mention their importance. 6. Define glycosides and mention biologically important examples. 7. State examples of homopolysaccharides and describe their structure and functions. 8. Classify glycosaminoglycans, mention their constituents and their biological importance. 9. Define proteoglycans and point out their functions. 10. Differentiate between glycoproteins and proteoglycans. CONTENTS: I. Chemical Nature of Carbohydrates II. Biomedical importance of Carbohydrates III. Monosaccharides - Classification - Forms of Isomerism of monosaccharides. - Importance of monosaccharides. - Monosaccharides derivatives. IV. Disaccharides - Reducing disaccharides. - Non- Reducing disaccharides V. Oligosaccarides. VI. Polysaccarides - Homopolysaccharides - Heteropolysaccharides - Carbohydrates of Biological Importance 10 CARBOHYDRATES OF BIOLOGICAL IMPORTANCE Chemical Nature of Carbohydrates Carbohydrates are polyhydroxyalcohols with an aldehyde or keto group. They are represented with general formulae Cn(H2O)n and hence called hydrates of carbons. -

Glycosidic Bond Or O-Glycosidic Bond, at Need
Seminar 4 Carbohydrates Definition Saccharides (glycids) are polyhydroxyaldehydes, polyhydroxyketones, or substances that give such compounds on hydrolysis 3 Classification Basal units Give monosaccharides when hydrolyzed MONOSACCHARIDES OLIGOSACCHARIDES POLYSACCHARIDES polyhydroxyaldehydes polyhydroxyketones 2 – 10 basal units polymeric GLYCOSES (sugars) GLYCANS water-soluble, sweet taste Don't use the historical misleading term carbohydrates, please. It was primarily derived from the empirical formula Cn(H2O)n and currently is taken as incorrect, not recommended in the IUPAC nomenclature (even though it can be found in numerous textbooks till now) 4 Saccharides • occur widely in the nature, present in all types of cells – the major nutrient for heterotrophs – energy stores (glycogen, starch) – components of structural materials (glycosaminoglycans) – parts of important molecules (nucleic acids, nucleotides, glycoproteins, glycolipids) – signalling function (recognition of molecules and cells, antigenic determinants) 5 Monosaccharides are simple sugars that cannot be hydrolyzed to simpler compounds Aldoses Ketoses Simple derivatives (polyhydroxyaldehydes) (polyhydroxyketones) modified monosaccharides are further classified according to the deoxysugars number of carbon atoms in their chains: amino sugars glyceraldehyde (a triose) dihydroxyacetone uronic acids tetroses tetruloses other simple derivatives pentoses pentuloses alditols hexoses hexuloses glyconic acids heptoses … heptuloses … glycaric acids Trivial names for stereoisomers glucose -

Oxidation of Isomaltose by Pseudomonas Taetrolens
JOURNAL OF BACrERIOLOoY, Aug. 1969, p. 623 Vol. 99, No. 2 Copyright 0 1969 American Society for Microbiology Printed In U.S.A. Oxidation of Isomaltose by Pseudomonas taetrolens M. STERNBERG AND L. B. LOCKWOOD Miles Laboratories, Inc., Elkhart, Indiana 46514 Received for publication 16 May 1969 Pseudomonas taetrolens NRRL B14 oxidized isomaltose without hydrolysis of the 1-6 glycosidic linkage. The resulting isomaltobionic acid was identified by chro- matographic studies of acid hydrolysates and reconversion to isomaltose. Pseudomonas graveolens NRRL B14 was shown Dowex 50 in the H+ form and the acids were to oxidize maltose and lactose to the correspond- absorbed on a column of Dowex 2X-4 in the ace- ing maltobionic and lactobionic acids, leaving tate form. A column (1,000 ml) of wet resin was intact the 1-4 glycosidic linkage (5). It was of used for 300 ml of filtered fermentation liquor. interest to determine whether the same organism, The column was eluted with a gradient of 0.1 to now named P. taetrolens, would oxidize an a 1-6 1 N acetic acid. Fractions of 200 ml were collected disaccharide, namely isomaltose, to isomalto- and assayed by the phenol sulfuric acid method bionic acid. (1). A major peak emerged between fractions 26 Isomaltose was prepared by the treatment of and 31. After evaporation at 40 to 42 C to 300 ml, maltose with transglucosidase. The enzyme was the concentrate was extracted three times with an obtained from a fermentation with Aspergillus equal volume of ethyl acetate to remove the acetic niger (3). After incubation of a 10% solution of acid and then was lyophilized. -

Effects of Sucrose Reduction on the Structural Characteristics of Sponge Cake1
Revista Ciência Agronômica, v. 46, n. 4, p. 718-723, out-dez, 2015 Centro de Ciências Agrárias - Universidade Federal do Ceará, Fortaleza, CE Artigo Científico www.ccarevista.ufc.br ISSN 1806-6690 Effects of sucrose reduction on the structural characteristics of sponge cake1 Efeitos da redução de açúcar nas características estruturais de bolos tipo esponja Rosane Souza Cavalcante2* e Claudio Ernani Mendes da Silva3 ABSTRACT - The consumption of reduced-calorie cakes has been increasing, however this has presented challenges to be overcome concerning the formation of their structure when the sucrose is substituted by alternative sweeteners, gums or thickening agents. The present study evaluated the internal characteristics of cakes with a reduction in sucrose, and the effects of its substitution on starch gelatinisation. Starting with a pre-established formulation, the sucrose was gradually substituted by a 1.0% mixture of sucralose in a 1.5% xanthan gum solution. In the substituted cake mix, the apparent viscosity and its thermal properties were evaluated using differential scanning calorimetry (DSC). Specific volume (SV) and cell count (CC) were evaluated in the cakes. As the sucrose content decreased (52.17 to 10.00%), the specific volume (1.94 to 0.7 mL/g), cell count (36.2 to 4.0 cell/cm²) and the apparent viscosity of the batter (337.56 to 631.40 cP) were also reduced. The results showed that substituting the sucrose contributed greatly to the formation of defects in the cake structure (holes). From the data obtained, and thermograms of standard cake batters and those with a reduction in sucrose, it can be concluded that sucralose reduced the temperature of starch gelatinisation, speeding the process and causing compaction of the cake structure during baking, favouring the formation of bubbles throughout the batter. -

19 Do Not Duplicate Functional Oligosaccharides a Trisaccharide Of
Do not duplicate Introduction to Carbohydrates: Oligosaccharides Dr. Yuan Yao Whistler Center for Carbohydrate Research Short Course October 3, 2017 Basic Concepts 2 Do not duplicate • “Oligo-” is the prefix from Greek language “few”; Poly- “many” • Oligosaccharides: Products of glycosidic linkages of 2-20 monosaccharide units (most commonly 2-9). Polysaccharides: More than 20 units • In the disaccharides: the aglycone is a monosaccharide unit; higher order oligosaccharides are named “tri-”, “tetra-”, “penta-”, etc. • There can be α-/β-(1→2), (1→3), (1→4) or (1→6) glycosidic linkages, with different stabilities & digestibilities (as for human body) • The structures of oligosaccharides could be linear or branched. Linear: head-to-tail linkage, 1 reducing end, 1 non-reducing end Branched: 1 reducing end, multiple non-reducing ends Common Disaccharides 3 Do not duplicate • Disaccharides are the simplest oligosaccharides that are only composed of two monosaccharide units o Highly abundant in nature; or the products of incomplete hydrolysis of higher oligosaccharides or polysaccharides o Water-soluble, with sweet taste • Most Common: Sucrose, Maltose, Lactose, & Trehalose o Naturally occurring o As the main product of photosynthesis, sucrose is ubiquitous in all plants, with high abundance in sugar cane and beet, as well as fruits o Commonly known as table sugar, sucrose usually serves as a “standard” of sweetness for other sweeteners Common Disaccharides 4 Do not duplicate Sucrose • A disaccharide of one glucose and one fructose unit, connected via β-(1,2)-glycosidic linkage • The “head-to-head” linkage is unstable due to high strain, and is therefore easily hydrolyzed (acid-catalyzed, or enzymatic) • Sucrose is a non-reducing sugar α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside 5 Common Disaccharides Inverted sugarDo (syrup) not duplicate • Sucrose could be readily hydrolyzed. -
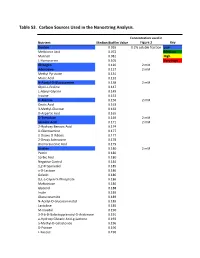
Supplementary Tables Vfinal.Xlsx
Table S3. Carbon Sources Used in the Nanostring Analysis. Concentration used in Nutrient Median Biofilm Value Figure 3 Key Dextrin 0.065 0.2% soluble fraction Low Melibionic Acid 0.072 Medium Mannan 0.081 High L-Homoserine 0.105 Very High Glycogen 0.120 2 mM Adenosine 0.127 2 mM Methyl Pyruvate 0.131 Mucic Acid 0.132 N-Acetyl-D-Glucosamine 0.138 2 mM Glycil-L-Proline 0.147 L-Alanyl-Glycine 0.149 Inosine 0.153 D-Alanine 0.156 2 mM Oxalic Acid 0.163 3-Methyl-Glucose 0.163 D-Aspartic Acid 0.165 D-Trehalose 0.169 2 mM Glycolic Acid 0.171 2 mM 2-Hydroxy Benzoic Acid 0.174 D-Glucosamine 0.177 2-Deoxy-D-Ribose 0.177 2-Deoxy Adenosine 0.178 Bromo Succinic Acid 0.179 Uridine 0.180 2 mM Pectin 0.180 Sorbic Acid 0.180 Negative Control 0.184 1,2-Propanediol 0.185 a-D-Lactose 0.186 Gelatin 0.186 D,L-a-Glycerol-Phosphate 0.186 Maltotriose 0.186 Glycerol 0.188 Inulin 0.189 Glucuronamide 0.189 N-Acetyl-D-Glucosaminatol 0.189 Lactulose 0.189 M-Inositol 0.190 3-0-b-D-Galactopyranosyl-D-Arabinose 0.191 a-Hydroxy Glutaric Acid-g-Lactone 0.193 a-Methyl-D-Galactoside 0.196 D-Psicose 0.196 L-Fucose 0.196 Succinamic Acid 0.197 D-Sorbitol 0.197 2 mM Dulcitol 0.198 D-Fructose-6-Phosphate 0.198 Sedoheptulosa 0.199 Glyoxylic Acid 0.200 Maltose 0.201 2 mM D-Ribono-1,4-Lactone 0.201 Sucrose 0.201 2 mM D-Melibiose 0.202 Mono Methyl Succinate 0.203 N-Acetyl-b-D-Mannosamine 0.204 p-Hydroxy Phenyl Acetic Acid 0.204 L-Rhamnose 0.205 2 mM D-Cellobiose 0.206 D-Glucuronic Acid 0.207 Adonitol 0.207 b-Methyl-D-Glucoside 0.207 Laminarin 0.208 Acetoacetic Acid 0.208 a-Keto-Glutaric