(12) Patent Application Publication (10) Pub. No.: US 2017/013538.6 A1 ARMENTROUT Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Honeydew Sugars with Respect to Their Utilization by the Hymenopteran Parasitoid Cotesia Glomerata F.L
Journal of Insect Physiology 47 (2001) 1077–1084 www.elsevier.com/locate/jinsphys A comparison of nectar- and honeydew sugars with respect to their utilization by the hymenopteran parasitoid Cotesia glomerata F.L. Wa¨ckers * Institute of Plant Sciences, Applied Entomology, Swiss Federal Institute of Technology (ETH), 8092 Zurich, Switzerland Received 10 October 2000; received in revised form 12 February 2001; accepted 19 February 2001 Abstract Fourteen naturally occurring sugars were individually tested with respect to their effect on Cotesia glomerata longevity. Parasitoids kept with solutions of either sucrose, glucose and fructose lived for Ͼ30 days. This constitutes a factor 15 increase in life span in comparison to control individuals kept with water only. Stachyose, mannose, melezitose, melibiose, maltose and erlose increased parasitoid longevity by a factor of 11.2–6.9. Solutions of galactose and trehalose had a marginal, but still significant effect. Lactose and raffinose did not raise parasitoid longevity, while rhamnose actually reduced parasitoid survival. In an additional experiment, the relationship between quantity of sugar consumption and longevity was established for all 14 sugars. To study the effect of an unsuitable sugar in sugar mixtures, a range of glucose:rhamnose mixtures was tested. Even at 20% of the sugar mixture rhamnose suppressed the nutritional benefit of the 80% glucose. The nutritional suitability of the sugars shows a positive correlation with the previously reported gustatory response towards the individual sugars. Patterns of sugar utilization are discussed with respect to hydrolytic enzymes and carbohydrate biochemical characteristics. Our findings for C. glomerata are compared to patterns of sugar utilization reported for other species. -

Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information Poly(ionic liquid)s as a Distinct Receptor Material to Create Highly- Integrated Sensing Platform for Efficiently Identifying a Myriad of Saccharides Wanlin Zhang, Yao Li, Yun Liang, Ning Gao, Chengcheng Liu, Shiqiang Wang, Xianpeng Yin, and Guangtao Li* *Corresponding authors: Guangtao Li ([email protected]) S1 Contents 1. Experimental Section (Page S4-S6) Materials and Characterization (Page S4) Experimental Details (Page S4-S6) 2. Figures and Tables (Page S7-S40) Fig. S1 SEM image of silica colloidal crystal spheres and PIL inverse opal spheres. (Page S7) Fig. S2 Adsorption isotherm of PIL inverse opal. (Page S7) Fig. S3 Dynamic mechanical analysis and thermal gravimetric analysis of PIL materials. (Page S7) Fig. S4 Chemical structures of 23 saccharides. (Page S8) Fig. S5 The counteranion exchange of PIL photonic spheres from Br- to DCA. (Page S9) Fig. S6 Reflection and emission spectra of spheres for saccharides. (Page S9) Table S1 The jack-knifed classification on single-sphere array for 23 saccharides. (Page S10) Fig. S7 Lower detection concentration at 10 mM of the single-sphere array. (Page S11) Fig. S8 Lower detection concentration at 1 mM of the single-sphere array. (Page S12) Fig. S9 PIL sphere exhibiting great pH robustness within the biological pH range. (Page S12) Fig. S10 Exploring the tolerance of PIL spheres to different conditions. (Page S13) Fig. S11 Exploring the reusability of PIL spheres. (Page S14) Fig. S12 Responses of spheres to sugar alcohols. (Page S15) Fig. -

Determination of Carbohydrates in Honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA
Determination of carbohydrates in honey Manali Aggrawal, Jingli Hu and Jeff Rohrer, Thermo Fisher Scientific, Sunnyvale, CA ABSTRACT RESULTS SAMPLE ANALYSIS METHOD ACCURACY Table 7. Adulteration parameters for HS6 adulterated with 10% SS1 through SS5. Purpose: To develop an HPAE-PAD method for the determination of carbohydrates in honey Honey sugar analysis Sample Recovery HS6 (Wild Mountain Honey) samples to evaluate their quality and to assess the possibility of adulteration. Separation Adulteration Honey sugars were separated using a Dionex CarboPac PA210-Fast-4μm column (150 × 4 mm) in Method accuracy was evaluated by measuring recoveries of 10 sugar standards spiked into honey Parameters 100% + 10% + 10% + 10% + 10% + 10% For this study, we purchased 12 commercial honey samples (Table 1) and analyzed them using Honey SS1 SS2 SS3 SS4 SS5 Methods: Separation of individual honey sugars was achieved on the recently introduced Thermo series with a Dionex CarboPac PA210 guard column (50 × 4 mm). The column selectivity allow samples. For spiking experiments, four honey samples were used (HS7–HS10) and spiked with a 10- HPAE-PAD. Figure 3 shows the representative chromatograms of 3 honey samples. For all 12 Glucose(G), mg/L 121 115 116 117 119 107 Scientific™ Dionex™ CarboPac™ PA210-Fast-4μm column. Carbohydrate detection was by pulsed carbohydrates to be separated with only a hydroxide eluent generated using an eluent generator. A sugar standard mix at two concentration levels. Figure 4 shows the representative chromatograms investigated honey samples, fructose and glucose (Peak 2 and Peak 3), were found to be the major Fructose(F), mg/L 127 115 115 116 126 116 amperometric detection (PAD) with a gold working electrode and, therefore, no sample derivatization solution of honey sugar standards was prepared and an aliquot (10 μL) of the solution was injected of unspiked and spiked honey sample HS7. -

Ep 3117827 A1
(19) TZZ¥__ _T (11) EP 3 117 827 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 18.01.2017 Bulletin 2017/03 A61K 31/7016 (2006.01) A61P 15/02 (2006.01) (21) Application number: 15760638.5 (86) International application number: PCT/CN2015/073978 (22) Date of filing: 11.03.2015 (87) International publication number: WO 2015/135470 (17.09.2015 Gazette 2015/37) (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • ZENG, Zhongming GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Shenzhen, Guangdong 518000 (CN) PL PT RO RS SE SI SK SM TR • ZHOU, Ruyun Designated Extension States: Shenzhen, Guangdong 518000 (CN) BA ME Designated Validation States: (74) Representative: Krauns, Christian MA Wallinger Ricker Schlotter Tostmann Patent- und Rechtsanwälte Partnerschaft mbB (30) Priority: 13.03.2014 CN 201410103662 Zweibrückenstraße 5-7 80331 München (DE) (71) Applicant: SINGAPORE ZE&Z INTERNATIONAL PTE. LTD. Singapore 079903 (SG) (54) COMPOSITION FOR VAGINA AND USE OF THE COMPOSITION (57) A use of isomaltulose in the preparation of a vag- non-therapeutic vaginal care method, a non-therapeutic inal composition, wherein the amount of isomaltulose vaginal cleaning method, a method for improving vaginal used is 0.05-20% (w/w). A vaginal composition contains acidity, a method for promoting growth of Lactobacillus 0.05-20% (w/w) of isomaltulose. The composition may in the vagina, and a method for preventing and treating be a health product, health care product, cleaning prod- vaginal dysbacteriosis, especially bacterial vaginal dis- uct, skin care product, deodorant, cosmetic, or disinfect- eases, these methods being implemented by adminis- ant composition or a pharmaceutical composition. -
Supplementary Tables Vfinal.Xlsx
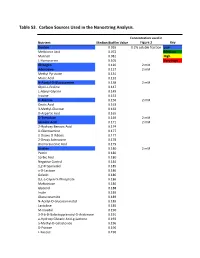
Table S3. Carbon Sources Used in the Nanostring Analysis. Concentration used in Nutrient Median Biofilm Value Figure 3 Key Dextrin 0.065 0.2% soluble fraction Low Melibionic Acid 0.072 Medium Mannan 0.081 High L-Homoserine 0.105 Very High Glycogen 0.120 2 mM Adenosine 0.127 2 mM Methyl Pyruvate 0.131 Mucic Acid 0.132 N-Acetyl-D-Glucosamine 0.138 2 mM Glycil-L-Proline 0.147 L-Alanyl-Glycine 0.149 Inosine 0.153 D-Alanine 0.156 2 mM Oxalic Acid 0.163 3-Methyl-Glucose 0.163 D-Aspartic Acid 0.165 D-Trehalose 0.169 2 mM Glycolic Acid 0.171 2 mM 2-Hydroxy Benzoic Acid 0.174 D-Glucosamine 0.177 2-Deoxy-D-Ribose 0.177 2-Deoxy Adenosine 0.178 Bromo Succinic Acid 0.179 Uridine 0.180 2 mM Pectin 0.180 Sorbic Acid 0.180 Negative Control 0.184 1,2-Propanediol 0.185 a-D-Lactose 0.186 Gelatin 0.186 D,L-a-Glycerol-Phosphate 0.186 Maltotriose 0.186 Glycerol 0.188 Inulin 0.189 Glucuronamide 0.189 N-Acetyl-D-Glucosaminatol 0.189 Lactulose 0.189 M-Inositol 0.190 3-0-b-D-Galactopyranosyl-D-Arabinose 0.191 a-Hydroxy Glutaric Acid-g-Lactone 0.193 a-Methyl-D-Galactoside 0.196 D-Psicose 0.196 L-Fucose 0.196 Succinamic Acid 0.197 D-Sorbitol 0.197 2 mM Dulcitol 0.198 D-Fructose-6-Phosphate 0.198 Sedoheptulosa 0.199 Glyoxylic Acid 0.200 Maltose 0.201 2 mM D-Ribono-1,4-Lactone 0.201 Sucrose 0.201 2 mM D-Melibiose 0.202 Mono Methyl Succinate 0.203 N-Acetyl-b-D-Mannosamine 0.204 p-Hydroxy Phenyl Acetic Acid 0.204 L-Rhamnose 0.205 2 mM D-Cellobiose 0.206 D-Glucuronic Acid 0.207 Adonitol 0.207 b-Methyl-D-Glucoside 0.207 Laminarin 0.208 Acetoacetic Acid 0.208 a-Keto-Glutaric -

Carbohydrate, Oligosaccharide, and Organic Acid Separations
Carbohydrate, Oligosaccharide, and Organic Acid Separations Long column lifetimes. Accurate, reproducible analysis. Trust Rezex™ For Excellent Resolution and Reproducibility of Sugars, Starches, and Organic Acids Phenomenex Rezex HPLC ion-exclusion columns are guaranteed to give you the performance you need. From drug formulation and excipient analysis to quality control testing of finished food products, Rezex columns consistently provide accurate and reproducible results. “ I have been using Phenomenex Rezex …for sugar quantitation in the last few years, we are very happy with the resolution of this column, plus a short running time. ” - Global Leader, Food & Beverage Ingredient Production Try Rezex Risk Free! Rezex is a guaranteed alternative to: • Bio-Rad® Aminex® If you are not completely satisfied with the performance Waters® Sugar-Pak™ • of any Rezex column, as compared to a competing Supelco® SUPELCOGEL™ product of the same size and phase, simply return the • Rezex column with your comparative data within 45 • Transgenomic® CARBOSep™ days for a FULL REFUND. Experience the Rezex™ Performance Advantage Broad Range of Phases to Perfectly Suit Your Application Needs .........................................2 Column Selection – Variety Gives You the Power of Optimization .................................................3 See the Difference! The Rezex Performance Advantage Sharper Peak Shape = Easy & Accurate Quantitation .........................................................4 Lower Backpressure = Longer Column Lifetimes & Faster -

Grasnotice 946, Lactobacillus Plantarum Strain DSM 33452
GRAS Notice (GRN) No. 946 https://www.fda.gov/food/generally-recognized-safe-gras/ RECEIVED gras-notice-inventory JUNO 2 1020 Chr. Hansen, In -?FFICE OF FOOD ADD ITIVE SAFETY 9015 West Maple Street Milwaukee, WI 53214 - 4298 Division of Biotechnology and GRAS Notice Review U.S .A. Center for Food Safety & Applied Nutrition (HFS-255) U.S. Food & Drug Administration Phone 414 - 607 - 5700 Fax 414 - 607 - 5959 Reference: Lactobacillus plantarum DSM 33452 June 1, 2020 Dear Sir or Madam, In accordance with the Federal Register [81 Fed. Reg. 159 (17 August 2016)] issuance on Generally Recognized as Safe (GRAS) notifications (21 CFR Part 170), Chr. Hansen is pleased to submit a notice that we have concluded, through scientific procedures that Lactobacillus plantarum (L. plantarum) DSM 33452 is generally recognized as safe for use in malolactic fermentation of wine and musts and is not subject to the pre-market approval requirements. The recommendation is to inoculate the pure starter culture of L. plantarum DSM 33452 into wine or must at an inoculation level of 1.0E+07 CFU/g at the time of crushing grapes or as early as possible in the fermentation tanks. L. plantarum DSM 33452 is sensitive to alcohol concentration, so the concentration of the organism will decrease as the concentration of alcohol in the wine or must increases. Though L. plantarum DSM 33452 is safe to consume, it would be present at negligible levels, if at all, in the finished product. It should also be noted that due to recent taxonomic changes to the genus Lactobaci/lus, Lactobacillus plantarum will be known as Lactiplantibacillus plantarum moving forward (Zheng, et al., 2020). -

(Mangifera Indica) Starch
fluids Article Rheological and Microstructural Properties of Oil-in-Water Emulsion Gels Containing Natural Plant Extracts Stabilized with Carboxymethyl Cellulose/Mango (Mangifera indica) Starch Luis Mieles-Gómez 1 , Santander E. Lastra-Ripoll 1 , Edilbert Torregroza-Fuentes 2, Somaris E. Quintana 1 and Luis A. García-Zapateiro 1,* 1 Research Group of Complex Fluid Engineering and Food Rheology, University of Cartagena, Cartagena 130015, Colombia; [email protected] (L.M.-G.); [email protected] (S.E.L.-R.); [email protected] (S.E.Q.) 2 Research Group Science Technology and Society—CTS, University of Cartagena, Cartagena 130015, Colombia; [email protected] * Correspondence: [email protected]; Tel.:+57-5-675-20-24 (ext. 205) Abstract: Emulsion gels are an alternative to developing food products and adding bioactive com- pounds; however, different stabilizers have been employed considering natural ingredients. In this work, stabilization of emulsion gels with blends of carboxymethylcellulose and kernel mango starch was performed with the addition of mango peel extracts, evaluating their physical, rheological and microstructural properties. Phenolic extract from mango peels (yields = 11.35 ± 2.05% w/w), with Citation: Mieles-Gómez, L.; 294.60 ± 0.03 mg GAE/100 g of extract and 436.77 ± 5.30 µMol Trolox/g of the extract, was obtained Lastra-Ripoll, S.E.; by ultrasound-assisted extraction (1:10 peel: Ethanol w/v, 200 W, 30 min), containing pyrogallol, Torregroza-Fuentes, E.; Quintana, melezitose, succinic acid, γ-tocopherol, campesterol, stigmasterol, lupeol, vitamin A and vitamin E. S.E.; García-Zapateiro, L.A. In addition, mango kernel starch (yields = 59.51 ± 1.35% w/w) with 27.28 ± 0.05% of amylose was Rheological and Microstructural Mangifera indica Properties of Oil-in-Water Emulsion obtained, being the by-product of mango ( var fachir) an alternative to producing Gels Containing Natural Plant natural food ingredients. -

An Investigation of Sugar Feeding by Black Flies (Diptera: Simuliidae)
An Investigation of Sugar Feeding by Black Flies (Diptera: Simuliidae) by Steve G. Burgin, B. Sc. A Thesis submitted to the Department of Biology in partial fulfillment of the requirements for the degree of Master of Biology Brock University St Catharines, Ontario © Steve G. Burgin, 1995 2 Abstract Although much research has been conducted on blood-meal acquisition in adult female black flies (Diptera: Simuliidae), the same cannot be said for sugar meals. Both sexes feed on sugar which provides energy for flight and it has been commonly held that nectar is the major carbohydrate source. This thesis addresses the question of whether a non-floral carbohydrate source, specifically homopteran honeydew, is ingested by male and female black flies. Black flies reared in the laboratory have been observed to readily ingest freshly excreted and older (dry) honeydew when presented with honeydew coated tamarack branches. Field work was conducted in Algonquin Park, Ontario in the spring and summer of 1993. Three separate studies were designed to test whether homopteran honeydew is an important carbohydrate source for black flies and whether flies from different habitats utilize different sugar sources. The sugars melezitose and / or stachyose are known to occur in a variety of homopteran honeydews and therefore were used as indicators of honeydew feeding by black flies. In the first study, black flies were collected with insect nets from a stand of Larix larcina heavily infested with honeydew - producing homopterans (Adelges lariciatus). Six black fly species were captured: Simulium venustum, S. rostratum, S. vittatum, Stegopterna mutata, S. aureum and S. quebecense. Samples of honeydew and individual black flies were tested using thin layer chromatography (T. -

Sucrose Metabolism and Exopolysaccharide Production by Lactobacillus Sanfranciscensis
Lehrstuhl für Technische Mikrobiologie Sucrose metabolism and exopolysaccharide production by Lactobacillus sanfranciscensis Maher Korakli Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. rer. nat. habil. S. Scherer Prüfer der Dissertation: 1. Univ.-Prof. Dr. rer. nat. habil. R. F. Vogel 2. Univ.-Prof. Dr. rer. nat. W. P. Hammes, Univ. Hohenheim 3. Univ.-Prof. Dr.-Ing. Dr.-Ing. habil. W. Back Die Dissertation wurde am 29.10.2002 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 9.12.2002 angenommen. Lehrstuhl für Technische Mikrobiologie Sucrose metabolism and exopolysaccharide production by Lactobacillus sanfranciscensis Maher Korakli Doctoral thesis Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt Freising 2002 Mein herzlicher Dank gilt: Meinem Doktorvater Prof. Rudi F. Vogel für die Überlassung des Themas, die zahlreichen Anregungen und die stete Bereitschaft zu fachlichen Diskussionen, für das mir entgegengebrachte Vertrauen und für den gewährten Raum meine Forschungsideen zu verwirklichen. Dr. Michael Gänzle für die motivierende und kritische Begleitung der Arbeit, die ständige Diskussionsbereitschaft und hilfreichen Anregungen. Monika Thalhammer, Nicole Kleber, Stephan Pröpsting, Melanie Pavlović, Konstanze Graser, Patrick Schwindt and allen fleißigen Händen für die fruchtbare Zusammenarbeit. Bedanken möchte ich mich weiterhin bei Angela Seppeur, Holger Schmidt, Georg Maier, Monika Hadek und Claudine Seeliger für die stete Hilfsbereitschaft. Darüber hinaus gilt mein Dank allen Mitarbeiterinnen und Mitarbeitern des Lehrstuhles für Technische Mikrobiologie für die kollegiale Zusammenarbeit und entspannte Laboratmosphäre. Contents 1. -

Rehydratable Matrices for Dry Storage of Taq
(19) TZZ Z_T (11) EP 2 440 658 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 9/96 (2006.01) C12N 9/12 (2006.01) 08.10.2014 Bulletin 2014/41 C12N 11/00 (2006.01) C12Q 1/68 (2006.01) B01L 3/00 (2006.01) (21) Application number: 10727592.7 (86) International application number: (22) Date of filing: 10.06.2010 PCT/US2010/038140 (87) International publication number: WO 2010/144682 (16.12.2010 Gazette 2010/50) (54) REHYDRATABLE MATRICES FOR DRY STORAGE OF TAQ POLYMERASE IN A MICROFLUIDIC DEVICE REHYDRATISIERBARE MATRIZEN FÜR TROCKENLAGERUNG VON TAQ-POLYMERASE IN EINER MIKROFLUIDISCHEN VORRICHTUNG MATRICES RÉHYDRATABLES POUR STOCKAGE À SEC DE LA TAQ POLYMÉRASE DANS UN DISPOSITIF MICROFLUIDIQUE (84) Designated Contracting States: (74) Representative: Grünecker, Kinkeldey, AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Stockmair & Schwanhäusser GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Leopoldstrasse 4 PL PT RO SE SI SK SM TR 80802 München (DE) (30) Priority: 12.06.2009 US 186441 P (56) References cited: WO-A1-91/18091 WO-A1-2008/036544 (43) Date of publication of application: US-A- 5 763 157 US-A1- 2002 173 016 18.04.2012 Bulletin 2012/16 US-A1- 2003 119 042 (73) Proprietor: Micronics, Inc. • RAMACHANDRAN S ET AL: "Dry-Reagent Redmond, WA 98052 (US) Storage for Disposable Lab-On-Card Diagnosis ofEnteric Pathogens" DISTRIBUTED DIAGNOSIS (72) Inventors: AND HOME HEALTHCARE, 2006. D2H2. 1ST • BATTRELL, C., Frederick TRANSDISCIP LINARY CONFERENCE ON Redmond MARRIOTT CRYSTAL GATEWAY HOTEL, Washington 98052 (US) ARLINGTON, VIRGINIA APRIL 2-4, 2006, • HOEKSTRA, Denise Maxine PISCATAWAY, NJ, USA, IEEE, NJ, US LNKD- DOI: Monroe 10.1109/DDHH.2006.1624786, 2 April 2006 Washington 98272 (US) (2006-04-02), pages 16-19, XP010912328 ISBN: • HAAB, Joan 978-1-4244-0058-4 Seattle Washington 98107 (US) Remarks: • WILLIFORD, John R. -

0975-8585 September – October 2016 RJPBCS 7(5) Page No. 2428
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Honey, Probiotics and Prebiotics: Review Bemmo Kamdem Ulrich Landry1, Sahoo Moumita2, R Jayabalan2*, and Zambou Ngoufack François1#. 1Laboratory of Biochemistry, Food Science and Nutrition (LABPMAN) - Department of Biochemistry - Faculty of Science - University of Dschang, P.O. Box 67 Dschang, Cameroon. 2Food Microbiology and Bioprocess Laboratory, Department of Life Science, National Institute of Technology, Rourkela 769 008, Odisha, India. ABSTRACT Nowadays, health awareness has risen to a great extent and hence non-dairy probiotic products are in high demand by consumers due to the major set-backs, allergy, and lactose intolerance, of the dairy based probiotic counterparts being commercialized. The functional attributes of such non-dairy probiotic products can be enhanced many fold by coalescing it with some natural prebiotic oligosaccharides. Prebiotics are substances which cannot be digested by pathogenic microorganisms and thereby selectively enhance the development of probiotics. Probiotics are live microorganisms which confer health-benefits in humans. Keeping this in view, the present review focuses on honey being an exceptional source of such prebiotic oligosaccharides (fructo-oligosaccharide, galacto-oligosaccharide) perfect for utilization to produce non-dairy synbiotic formulations. Honey is a natural food consumed by humans since many centuries by almost all civilizations due to its rich nutritional and therapeutic values. It remains stable at room temperature for long time. The complex nature of honey does not favor the development of majority of microorganisms. However, honey has been reported to stimulate the growth and/or activity of probiotic microorganisms due to its prebiotic content. Keywords: Honey, Probiotic, Prebiotic, Synbiotic, Oligosaccharides *Corresponding author, #Co-corresponding author September – October 2016 RJPBCS 7(5) Page No.