TOXICOLOGICAL REVIEW of HEXAVALENT CHROMIUM (CAS No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protective Effect of Melatonin Against Chromium-Induced Hepatotoxic and Genotoxic Effect in Albino Rats
Global Veterinaria 16 (4): 323-329, 2016 ISSN 1992-6197 © IDOSI Publications, 2016 DOI: 10.5829/idosi.gv.2016.16.04.10332 Protective Effect of Melatonin Against Chromium-Induced Hepatotoxic and Genotoxic Effect in Albino Rats Emad A. Hashish and Shimaa A. Elgaml Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Sharkyia, Egypt Abstract: Chromium is a widespread environmental waste. It is an industrial contaminant with teratogenic, mutagenic and carcinogenic effects on animals and human. This study was carried out to evaluate the potential protective effect of melatonin on the hepatotoxicity and genotoxicity generated by potassium dichromate (K2 Cr 27 O ) in albino rats. Rats were divided into four groups; control, melatonin (10 mg/kg b.wt.), melatonin pretreated with single S/C injection of K2 Cr 27 O (15 mg/kg b.wt.) and potassium dichromate treated group. Rats were sacrificed 24 h after K2 Cr 27 O treatment. K2 Cr 27 O treated rats showed significant (P<0.05) increase in the hepatic marker enzymes activity (aspartate aminotransferase-AST and alanine aminotransferase-ALT) and serum bilirubin (total and direct) was detected. Significant (P<0.05) decrease in the serum total protein and albumin was observed after K2 Cr 27 O treatment. Meanwhile, serum globulin, A/G ratio and indirect bilirubin showed no significant change. Hepatic DNA damage was observed using comet assay. The histological alterations confirmed the previous results. Melatonin pretreated rats showed an amelioration of the adverse effects of K2 Cr 27 O toxicity. An improvement in the serum hepatic enzymes (AST and ALT), proteinogram (total protein and albumin) and bilirubin (total and direct) was observed. -

Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation
sustainability Review Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation Dipali Srivastava 1,†, Madhu Tiwari 1,†, Prasanna Dutta 1,2, Puja Singh 1,2, Khushboo Chawda 1,2, Monica Kumari 1,2 and Debasis Chakrabarty 1,2,* 1 Biotechnology and Molecular Biology Division, CSIR-National Botanical Research Institute, Lucknow 226001, India; [email protected] (D.S.); [email protected] (M.T.); [email protected] (P.D.); [email protected] (P.S.); [email protected] (K.C.); [email protected] (M.K.) 2 Academy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Extensive industrial activities resulted in an increase in chromium (Cr) contamination in the environment. The toxicity of Cr severely affects plant growth and development. Cr is also recognized as a human carcinogen that enters the human body via inhalation or by consuming Cr-contaminated food products. Taking consideration of Cr enrichment in the environment and its toxic effects, US Environmental Protection Agency and Agency for Toxic Substances and Disease Registry listed Cr as a priority pollutant. In nature, Cr exists in various valence states, including Cr(III) and Cr(VI). Cr(VI) is the most toxic and persistent form in soil. Plants uptake Cr through various transporters such as phosphate and sulfate transporters. Cr exerts its effect by generating reactive oxygen species (ROS) and hampering various metabolic and physiological pathways. Studies Citation: Srivastava, D.; Tiwari, M.; on genetic and transcriptional regulation of plants have shown the various detoxification genes get Dutta, P.; Singh, P.; Chawda, K.; up-regulated and confer tolerance in plants under Cr stress. -
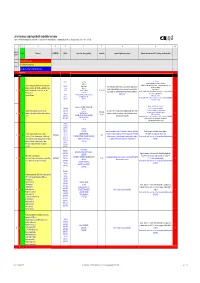
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Hexavalent Chromium (Cr VI) in Drinking Water
PUBLIC HEALTH GOALS FOR CHEMICALS IN DRINKING WATER HEXAVALENT CHROMIUM (Cr VI) July 2011 Governor of the State of California Edmund G. Brown Jr. Acting Secretary for Environmental Protection California Environmental Protection Agency Linda S. Adams Acting Director Office of Environmental Health Hazard Assessment George V. Alexeeff, Ph.D. Public Health Goal for Hexavalent Chromium (Cr VI) in Drinking Water Prepared by Pesticide and Environmental Toxicology Branch Office of Environmental Health Hazard Assessment California Environmental Protection Agency July 2011 PREFACE Drinking Water Public Health Goals Pesticide and Environmental Toxicology Branch Office of Environmental Health Hazard Assessment California Environmental Protection Agency This Public Health Goal (PHG) technical support document provides information on health effects from contaminants in drinking water. PHGs are developed for chemical contaminants based on the best available toxicological data in the scientific literature. These documents and the analyses contained in them provide estimates of the levels of contaminants in drinking water that would pose no significant health risk to individuals consuming the water on a daily basis over a lifetime. The California Safe Drinking Water Act of 1996 (Health and Safety Code, Section 116365) requires the Office of Environmental Health Hazard Assessment (OEHHA) to perform risk assessments and adopt PHGs for contaminants in drinking water based exclusively on public health considerations. The Act requires that PHGs be set in accordance with the following criteria: 1. PHGs for acutely toxic substances shall be set at levels at which no known or anticipated adverse effects on health will occur, with an adequate margin of safety. 2. PHGs for carcinogens or other substances that may cause chronic disease shall be based solely on health effects and shall be set at levels that OEHHA has determined do not pose any significant risk to health. -

Nutrition Journal of Parenteral and Enteral
Journal of Parenteral and Enteral Nutrition http://pen.sagepub.com/ Micronutrient Supplementation in Adult Nutrition Therapy: Practical Considerations Krishnan Sriram and Vassyl A. Lonchyna JPEN J Parenter Enteral Nutr 2009 33: 548 originally published online 19 May 2009 DOI: 10.1177/0148607108328470 The online version of this article can be found at: http://pen.sagepub.com/content/33/5/548 Published by: http://www.sagepublications.com On behalf of: The American Society for Parenteral & Enteral Nutrition Additional services and information for Journal of Parenteral and Enteral Nutrition can be found at: Email Alerts: http://pen.sagepub.com/cgi/alerts Subscriptions: http://pen.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Aug 27, 2009 OnlineFirst Version of Record - May 19, 2009 What is This? Downloaded from pen.sagepub.com by Karrie Derenski on April 1, 2013 Review Journal of Parenteral and Enteral Nutrition Volume 33 Number 5 September/October 2009 548-562 Micronutrient Supplementation in © 2009 American Society for Parenteral and Enteral Nutrition 10.1177/0148607108328470 Adult Nutrition Therapy: http://jpen.sagepub.com hosted at Practical Considerations http://online.sagepub.com Krishnan Sriram, MD, FRCS(C) FACS1; and Vassyl A. Lonchyna, MD, FACS2 Financial disclosure: none declared. Preexisting micronutrient (vitamins and trace elements) defi- for selenium (Se) and zinc (Zn). In practice, a multivitamin ciencies are often present in hospitalized patients. Deficiencies preparation and a multiple trace element admixture (containing occur due to inadequate or inappropriate administration, Zn, Se, copper, chromium, and manganese) are added to par- increased or altered requirements, and increased losses, affect- enteral nutrition formulations. -

Sd0000029 Evaluation of Chromfte Ore and The
SD0000029 EVALUATION OF CHROMFTE ORE AND THE OPTIMUM METHODS FOR INDUSTRIAL EXTRACTION OF CHROMIUM A TI1HSIS SUBMITTED BY Bakheit Mustafa Mohamed Salih IN CANDIDATURE FOR TIU:DI:GRFJ:OI MASTER OF SC1HNCT DEPARTMENT OF CHEMISTRY FACULTY OF SCIENCE (P.O.BOX 321) OCTOBER 1999 UNIVERSITY OF KHARTOUM 31/ 28 ABSTRACT Samples of chromite ore, collected from Gam and Cheikay mining area (Ingessana Hills) in east Sudan, were analysed to assess the chromium content. Methods for extraction and analysis of chromium metal were developed and established. Analysis were carried out using atomic absorption spectroscopy (AAS) to estimate the contents of chromium, iron, calcium, and magnesium. X-ray fluorescence (XRF) was used to evaluate the levels of chromium, iron, and calcium in the ore. Volumetric analysis was performed to assess chromium and iron, whilst gravimetric analysis was employed to measure the amounts of calcium, magnesium, aluminum and silicon present in the ore. The data was chemically and statistically analyzed to compare the results obtained by the given analytical methods. The results are in good agreement except iron oxide, which displayed a significantly different value when measured by x-ray fluorescence. The data obtained exhibited similarity in almost all cases, when compared with local and global researches, reports, and literature. The study has revealed the average contents of Cr2O3, FeO, CaO, MgO, A12O3, and SiO2 as 40.66. 11.96, 11.94. 0.36. 16.94, 11.45% respectively. MnO and NiO were detected in trace amounts, the corresponding levels in the ore being 72 and 27 ppm. The average chromium content in extracted potassium dichromate measured by using AAS, XRF, and volumetric methods was found to be 31.7%. -

Potassium Dichromate CAS Number: 7778-50-9 EC Number: 231-906-6
21.5.2010 COMMENTS AND RESPONSE TO COMMENTS ON ANNEX XV SVHC: PROPOSAL AND JUSTIFICATION Substance name: Potassium dichromate CAS number: 7778-50-9 EC number: 231-906-6 Reason of the submission of the Annex XV: CMR Disclaimer: The European Chemicals Agency is not responsible for the content of this document. The Response to Comments table has been prepared by the competent authority of the Member State preparing the proposal for identification of a Substance of Very High Concern. The comments were received during the public consultation of the Annex XV dossier. General comments Date Submitted by (name, Comment Response Organisation/MSCA) 20100323 CLEAPSS, UK (on behalf of an CLEAPSS is an advisory service providing support in No answer expected. organisation) science and technology for a consortium of local authorities and their schools including establishments for pupils with special needs. Independent schools, post-16 colleges, teacher training establishments, curriculum developers and others can apply for associate membership. We offer help from nursery education through to A-level studies or equivalent. Our services cover health & safety, risk assessment, sources and use of chemicals, living organisms and equipment. CLEAPSS also provides advice on technicians & their jobs, as well as the design of laboratories and facilities & fittings for D&T and science rooms. 20100324 Germany, company (on behalf of an We reject the ban of Potassium Dichromate in general, Thank for your comment. organisation) because the substance is used in a low concentration (< 1%) in an almost closed system of cuvette tests 20100408 Draeger Safety AG & Co. KGaA, comments k2Cr2O7R1.doc Thank you for this information. -

Rpt POL-TOXIC AIR POLLUTANTS 98 BY
SWCAA TOXIC AIR POLLUTANTS '98 by CAS ASIL TAP SQER CAS No HAP POLLUTANT NAME HAP CAT 24hr ug/m3 Ann ug/m3 Class lbs/yr lbs/hr none17 BN 1750 0.20 ALUMINUM compounds none0.00023 AY None None ARSENIC compounds (E649418) ARSENIC COMPOUNDS none0.12 AY 20 None BENZENE, TOLUENE, ETHYLBENZENE, XYLENES BENZENE none0.12 AY 20 None BTEX BENZENE none0.000083 AY None None CHROMIUM (VI) compounds CHROMIUM COMPOUN none0.000083 AY None None CHROMIUM compounds (E649962) CHROMIUM COMPOUN none0.0016 AY 0.5 None COKE OVEN COMPOUNDS (E649830) - CAA 112B COKE OVEN EMISSIONS none3.3 BN 175 0.02 COPPER compounds none0.67 BN 175 0.02 COTTON DUST (raw) none17 BY 1,750 0.20 CYANIDE compounds CYANIDE COMPOUNDS none33 BN 5,250 0.60 FIBROUS GLASS DUST none33 BY 5,250 0.60 FINE MINERAL FIBERS FINE MINERAL FIBERS none8.3 BN 175 0.20 FLUORIDES, as F, containing fluoride, NOS none0.00000003 AY None None FURANS, NITRO- DIOXINS/FURANS none5900 BY 43,748 5.0 HEXANE, other isomers none3.3 BN 175 0.02 IRON SALTS, soluble as Fe none00 AN None None ISOPROPYL OILS none0.5 AY None None LEAD compounds (E650002) LEAD COMPOUNDS none0.4 BY 175 0.02 MANGANESE compounds (E650010) MANGANESE COMPOU none0.33 BY 175 0.02 MERCURY compounds (E650028) MERCURY COMPOUND none33 BY 5,250 0.60 MINERAL FIBERS ((fine), incl glass, glass wool, rock wool, slag w FINE MINERAL FIBERS none0.0021 AY 0.5 None NICKEL 59 (NY059280) NICKEL COMPOUNDS none0.0021 AY 0.5 None NICKEL compounds (E650036) NICKEL COMPOUNDS none0.00000003 AY None None NITROFURANS (nitrofurans furazolidone) DIOXINS/FURANS none0.0013 -

Chromate and the Environment: Removal and Utilization of Industrial Waste
J. Chem. Chem. Eng. 10 (2016) 147-152 doi: 10.17265/1934-7375/2016.03.006 D DAVID PUBLISHING Chromate and the Environment: Removal and Utilization of Industrial Waste Fernando B. Mainier, Pedro Paulo B. Leite, Marcone F. Reis and Thiago Teobaldo Silva Engineering School, Federal Fluminense University(UFF), Niterói, Rio de Janeiro 24220-261, Brazil Abstract: Chromate and dichromate sodium as a function of oxidizer characteristics are used in several industrial areas; for example, in surface protection of coated parts of cadmium, zinc and aluminum (chromate coated treated), corrosion inhibitors, the treatment of leather, the manufacture of pigments, etc. However, the use of such products has been questioned due to the problems of toxicity and pollution that can be caused in the environmental. The Brazilian environmental agency has established that the concentrations of 2- chromate in water courses are less than 0.5 ppm. In order to reuse chromate (CrO4 ) from industrial effluent, laboratory experiments have been proposed based on chemical reduction or electrolytic processes, in order to transform these chromate ions in a final mix of oxides (in solid form), which can then be packed and sent to the production process of sodium chromate. The results of these experiments have become useful industrially (without regard to costs) considering the environmental reuse and the life cycle of the chemical compound. Key words: Chromate, dichromate, contamination, chemical reduction, electrolytic process. 1. Introduction waste, among others [1, 2]. In -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

CHROMIUM and Ehromium Eompounds
CHROMIUM AND eHROMIUM eOMPOUNDS Chromium and chromium compounds were considered by previous IAC Working Groups, in 1972, 1979, 1982 and 1987 (lARe, 1973, 1979, 1980a, 1982, 1987a). Since that time, new data have become available, and these are included in the present monograph and have been taken into consideration in the evaluation. 1. ehemical and Physical Data The list of chromium alloys and compounds given in Table 1 is not exhaustive, nor does it necessarily reflect the commercial importance of the various chro- mium-containing substances, but it is indicative of the range of chromium alloys and compounds available. 1.1 Synonyms, trade names and molecular formulae of chromium and selected chromium-containing compouDds Table 1. Synonyms (Chemical Abstracts Service names are given iD bold), trade names and atomic or molecular formulae of chromium and selected chromium compounds Chemical Chem. Abstr. Synonyms and trade names Formulab na me Seivices Reg. No. a Metallc chromium (0) and chromium (0) alloys Chromium 7440-47-3 Chrome Cr Cobalt-chro- 11114-92-4 Chromium alloy (nonbase), Co, Cr; cobalt alloy (non- mium alloyC (91700-55-9) base), Co, Cr Cobalt-chro- 12629-02.; Cobalt alloy (base), Co 56-68, Cr 25-29, Mo 5-6, Ni mium-molyb- (8064-15-1; 1.8-3.8, Fe 0-3, Mn 0-1, Si 0-1, C 0.2-0.3 (ASTM A567-1) denum 11068-92-1; alloyC 12618-69-8; 55345-18- 1; -49- 50 lARe MONOGRAHS VOLUME 49 Table 1 (contd) Chernical Chern. Abstr. Synonyrs and trade narnes Forrulab narne Servces Reg. -

Chemobiokinetics, Biotoxicity and Therapeutic Overview of Selected Heavy Metals Poisoning: a Review
Biodiversity International Journal Review Article Open Access Chemobiokinetics, biotoxicity and therapeutic overview of selected heavy metals poisoning: a review Abstract Volume 4 Issue 5 - 2020 Numerous debates exist as to the precise definition of the term “heavy metal” and which SOKAN-ADEAGA Adewale Allen,1 SOKAN- elements appropriately fit into such. Several authors rationalized the definition on atomic ADEAGA Micheal Ayodeji,2 SOKAN- weight; others, based on specific gravity of greater than 4.0, or more than 5.0 while a few 3 based it on chemical behaviour. Regardless of one’s choice of classification, heavy metal ADEAGA Eniola Deborah, OKAREH Titus 1 4 toxicity is a rare diagnosis. However, if undetected or inefficiently managed, heavy metal Oladapo, EDRIS Hoseinzadeh exposure can lead to remarkably disability and death. This paper gives a succinct and 1Department of Environmental Health Sciences, Faculty of systematic review on the emission, absorption, metabolism and excretion of selected heavy Public Health, College of Medicine, University of Ibadan, Ibadan, metals. It also delves into their biotoxic effects on the human wellbeing and the ecosystem Nigeria 2Department of Community Health and Primary Health Care, in general with the mechanisms of their actions. It concludes with the various therapeutic Faculty of Clinical Sciences, College of Medicine, University of options and management plans for different heavy metal poisoning. This review posits Lagos, Lagos, Nigeria that though heavy metal poisoning could be clinically diagnosed and medically treated, 3Department of Physiology, Faculty of Basic Medical Sciences, the most appropriate measure is to avoid heavy metal contamination and its subsequent College of Medicine, Ladoke Akintola University of Technology human exposure and toxicity.