Sd0000029 Evaluation of Chromfte Ore and The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Podiform Chromite Deposits—Database and Grade and Tonnage Models
Podiform Chromite Deposits—Database and Grade and Tonnage Models Scientific Investigations Report 2012–5157 U.S. Department of the Interior U.S. Geological Survey COVER View of the abandoned Chrome Concentrating Company mill, opened in 1917, near the No. 5 chromite mine in Del Puerto Canyon, Stanislaus County, California (USGS photograph by Dan Mosier, 1972). Insets show (upper right) specimen of massive chromite ore from the Pillikin mine, El Dorado County, California, and (lower left) specimen showing disseminated layers of chromite in dunite from the No. 5 mine, Stanislaus County, California (USGS photographs by Dan Mosier, 2012). Podiform Chromite Deposits—Database and Grade and Tonnage Models By Dan L. Mosier, Donald A. Singer, Barry C. Moring, and John P. Galloway Scientific Investigations Report 2012-5157 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 This report and any updates to it are available online at: http://pubs.usgs.gov/sir/2012/5157/ For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Suggested citation: Mosier, D.L., Singer, D.A., Moring, B.C., and Galloway, J.P., 2012, Podiform chromite deposits—database and grade and tonnage models: U.S. -

Chromite Crystal Structure and Chemistry Applied As an Exploration Tool
Western University Scholarship@Western Electronic Thesis and Dissertation Repository February 2015 Chromite Crystal Structure and Chemistry applied as an Exploration Tool Patrick H.M. Shepherd The University of Western Ontario Supervisor Dr. Roberta L. Flemming The University of Western Ontario Graduate Program in Geology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Patrick H.M. Shepherd 2015 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Geology Commons Recommended Citation Shepherd, Patrick H.M., "Chromite Crystal Structure and Chemistry applied as an Exploration Tool" (2015). Electronic Thesis and Dissertation Repository. 2685. https://ir.lib.uwo.ca/etd/2685 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Western University Scholarship@Western University of Western Ontario - Electronic Thesis and Dissertation Repository Chromite Crystal Structure and Chemistry Applied as an Exploration Tool Patrick H.M. Shepherd Supervisor Roberta Flemming The University of Western Ontario Follow this and additional works at: http://ir.lib.uwo.ca/etd Part of the Geology Commons This Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in University of Western Ontario - Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Chromite Crystal Structure and Chemistry Applied as an Exploration Tool (Thesis format: Integrated Article) by Patrick H.M. -

How to Handle Cryogenic Liquids
Photographic Processing Hazards, by Michael McCann, Ph.D., CIH • Black-and-White Photographic Processing o Mixing Photochemicals o Developing Baths o Stop Baths and Fixers o Intensifiers and Reducers o Toners o Other Hazards • Color Processing o Developing Baths o Bleaching, Fixing, and Other Steps Black-and-White Photographic Processing A wide variety of chemicals are used in black and white photographic processing. Film developing is usually done in closed canisters. Print processing uses tray processing, with successive developing baths, stop baths, fixing baths, and rinse steps. Other treatments include use of hardeners, intensifiers, reducers, toners, and hypo eliminators. Mixing Photochemicals Photochemicals can be bought in liquid form, which only need diluting, or powder form, which need dissolving and diluting. Hazards 1. Developer solutions and powders are often highly alkaline, and glacial acetic acid, used in making the stop bath, is also corrosive by skin contact, inhalation and ingestion. 2. Developer powders are highly toxic by inhalation, and moderately toxic by skin contact, due to the alkali and developers themselves (see Developing Baths below). Precautions 1. Use liquid chemistry whenever possible, rather than mixing developing powders. Pregnant women, in particular, should not be exposed to powdered developer. 2. When mixing powdered developers, use a glove box (a cardboard box with glass or plexiglas top, and two holes in the sides for hands and arms), local exhaust ventilation, or wear a NIOSH-approved toxic dust respirator. 3. Wear gloves, goggles and protective apron when mixing concentrated photochemicals. Always add any acid to water, never the reverse. 4. In case of skin contact, rinse with lots of water. -

Spinel Group Minerals in Metamorphosed Ultramafic Rocks from Río De Las Tunas Belt, Central Andes, Argentina
Geologica Acta, Vol.11, Nº 2, June 2013, 133-148 DOI: 10.1344/105.000001836 Available online at www.geologica-acta.com Spinel group minerals in metamorphosed ultramafic rocks from Río de Las Tunas belt, Central Andes, Argentina 1 1 2 M.F. GARGIULO E.A. BJERG A. MOGESSIE 1 INGEOSUR (Universidad Nacional del Sur – CONICET) San Juan 670, B8000ICN Bahía Blanca, Argentina Gargiulo E-mail: [email protected]; [email protected] Bjerg E-mail: [email protected] 2 Institut für Erdwissenschaften, Bereich Mineralogie und Petrologie, Karl-Franzens Universität Graz Universitätsplatz 2, 8010 Graz, Austria E-mail: [email protected] ABS TRACT In the Río de Las Tunas belt, Central Andes of Argentina, spinel group minerals occur in metaperidotites and in reaction zones developed at the boundary between metaperidotite bodies and their country-rocks. They comprise two types: i) Reddish-brown crystals with compositional zonation characterized by a ferritchromite core surrounded by an inner rim of Cr-magnetite and an outer rim of almost pure magnetite. ii) Green crystals chemically homogeneous with spinel (s.s.) and/or pleonaste compositions. The mineral paragenesis Fo+Srp+Cln+Tr+Fe-Chr and Fo+Cln+Tr+Tlc±Ath+Fe-Chr observed in the samples indicate lower and middle grade amphibolite facies metamorphic conditions. Nonetheless, the paragenesis (green)Spl+En+Fo±Di indicates that granulite facies conditions were also reached at a few localities. Cr-magnetite and magnetite rims in zoned reddish-brown crystals and magnetite rims around green-spinel/pleonaste grains are attributed to a later serpentinization process during retrograde metamorphism. -

Chromium(VI) and Oxyanion Remediation of Vadose Zone Soils with Zero Valent Iron (ZVI) and Biological Reduction
UNLV Theses, Dissertations, Professional Papers, and Capstones 5-1-2019 Chromium(VI) and Oxyanion Remediation Of Vadose Zone Soils With Zero Valent Iron (ZVI) and Biological Reduction Nicolas Kim Wong Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Environmental Engineering Commons Repository Citation Wong, Nicolas Kim, "Chromium(VI) and Oxyanion Remediation Of Vadose Zone Soils With Zero Valent Iron (ZVI) and Biological Reduction" (2019). UNLV Theses, Dissertations, Professional Papers, and Capstones. 3703. http://dx.doi.org/10.34917/15778575 This Thesis is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. CHROMIUM(VI) AND OXYANION REMEDIATION OF VADOSE ZONE SOILS WITH ZERO VALENT IRON (ZVI) AND BIOLOGICAL REDUCTION By Nicolas Wong Bachelor of Science in Engineering – Civil and Environmental Engineering Bachelor of Science – Geology University of Nevada, Las Vegas 2014 A thesis submitted in partial fulfillment of the requirements for the Master of Science in Civil Engineering – Civil and Environmental Engineering Department of Civil and Environmental Engineering and Construction Howard R. -
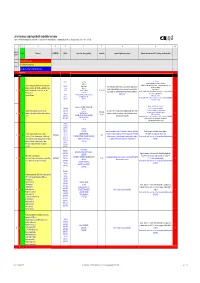
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Massachusetts Chemical Fact Sheet
Massachusetts Chemical Fact Sheet Hexavalent Chromium Table 1: HEXAVALENT CHROMIUM COMPOUNDS: Compounds SELECTED EXAMPLES* Compound Chemical Formula CAS # This fact sheet is part of a series of chemical fact sheets Ammonium chromate (NH ) Cr0 7788-98-9 developed by TURI to help Massachusetts companies, 4 2 4 community organizations and residents understand the Ammonium dichromate (NH4)2Cr2O7 7789-09-5 chemical’s use and health and environmental effects, as Barium chromate BaCrO4 10294-40-3 well as the availability of safer alternatives. tert-Butyl Chromate [(CH3)3CO]2CrO2 1189-85-1 Hexavalent chromium compounds are a toxic form of Calcium chromate CaCrO4 13765-19-0 chromium and are used in a variety of industrial processes Chromic acid H2CrO4 7738-94-5 and products. Chromium VI chloride CrCl6 14986-48-2 Hexavalent chromium compounds are human carcinogens, Chromic trioxide CrO3 1333-82-0 mutagens and developmental toxicants and are acutely Hexavalent chromium ion Cr6+ 18540-29-9 toxic. Non-hexavalent chromium compounds do not pose Lead chromate PbCrO4 7758-97-6 the same level of concern with regard to either chronic or Lead chromate oxide PbCrO4-PbO 8454-12-1 acute toxicity. Potassium chlorochromate KCrO3Cl 16037-50-6 Until 2011, all chromium compounds were treated as Potassium chromate K2CrO4 7789-00-6 a single category under TURA. Beginning with Potassium dichromate K Cr O 7778-50-9 reporting year 2012, hexavalent chromium 2 2 7 compounds are reportable under TURA as a Silver chromate Ag2CrO4 7784-01-2 separate category and are designated as a Higher Sodium chromate Na2CrO4 7775-11-3 Hazard Substance, which lowers the reporting Sodium dichromate 7789-12-0 threshold to 1,000 lb/year. -

Biophysical Characterization of Protein Interactions by ITC and NMR Xiaochen Lin University of Connecticut - Storrs, [email protected]
University of Connecticut OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 4-29-2016 Biophysical Characterization of Protein Interactions by ITC and NMR Xiaochen Lin University of Connecticut - Storrs, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Lin, Xiaochen, "Biophysical Characterization of Protein Interactions by ITC and NMR" (2016). Doctoral Dissertations. 1058. https://opencommons.uconn.edu/dissertations/1058 Biophysical Characterization of Protein Interactions by ITC and NMR Xiaochen Lin, PhD University of Connecticut, 2016 Proteins are crucial in virtually all cell activities. The vast majority of proteins interact with other molecules to function properly. The investigation of biomolecular interactions calls for structural and thermodynamic information acquired by a variety of experimental techniques and analytical methods. This dissertation is mainly concentrated on two frequently used and powerful tools, Isothermal Titration Calorimetry (ITC) and Nuclear Magnetic Resonance (NMR) spectroscopy, to investigate protein interactions. p52 Shc, a well-studied cellular adaptor protein, is a positive regulator in the mitogen-activated protein kinases pathway. We have confirmed that Shc interacts with both, integrin tyrosine(s)-phosphorylated cytoplasmic tails and phosphotidylinositol lipids, through the same Phospho-Tyrosine Binding (PTB) domain. The binding to phosphorylated peptides is enthalpy driven and tighter, while Shc interactions with PtdIns are entropy driven and much weaker. Shc interactions with PtdIns and β3-derived peptides are only weakly competitive with partially overlapping bindings sites. We also observed thermodynamic indications for potential intramolecular interactions within Shc, which could be perturbed by the binding to the phosphorylated receptor. BTN3A1, an immunoglobulin superfamily member, was found recently to be critical in Vγ9Vδ2 T cell activation. -

Conversion of Chromium Ore Processing Residue to Chrome Steel
Conversion of Chromium Ore Processing Residue to Chrome Steel Final Report Submitted by Dr. Jay N. Meegoda, P.E. Dr. Zhengbo Hu and Dr. Wiwat Kamolpornwijit Dept. of Civil & Environmental Engineering New Jersey Institute of Technology Newark, NJ 07102 For the New Jersey Department of Environmental Protection NJDEP Project Manager: Mr. Robert T. Mueller December 2007 TABLE OF CONTENTS Introduction 1 Literature Search 3 Experimental Program 15 Results and Discussion 23 Summary and Conclusions 42 Acknowledgements 43 References 44 ii Conversion of Chromium Ore Processing Residue to Chrome Steel Introduction Chromium played an important role in the industrial development of New Jersey from 1905 to 1971. During that period, chromate (Cr6+) was produced from chromite ore at three facilities in Hudson County, NJ. During the chromate extraction process, varying amounts of lime and soda ash were added and roasted with pulverized chromite ore to a temperature between 1100ºC and 1150ºC under an oxidizing environment. Trivalent chromium in chromite ore was oxidized to hexavalent chromium. The highly soluble hexavalent chromium was then removed from the COPR (left over Chromium Ore Processing Residue) leaving un-oxidized trivalent chromium and slow-dissolving hexavalent chromium compounds [Burke et al., 1991]. In the absence of information on the toxicity of hexavalent chromium, COPR was subsequently used for the back- filling of demolition sites, preparation for building foundations, construction of tank berms, roadway construction, the filling of wetlands, and other construction and development related purposes. The US Environmental Protection Agency (EPA) has classified hexavalent chromium as a Group A Human Carcinogen. Some forms of hexavalent chromium are water soluble in the full pH range, while trivalent chromium tends to be absorbed onto COPR sample surface or precipitate as chromium hydroxide in slightly acidic and alkaline environment. -

Art Studio Safety Manual
Art Studio Safety Manual St. Louis Community College Manual prepared on Jan 27, 2016 by the STLCC EHS Specialist, John Snider CHMM (Certified Hazardous Material Manager), CIHT (Certified Industrial Hygiene Technician), Registered Radiation Protection Technologist (RRPT) Art Safety Manual The visual arts can pose Chemicals, compressed significant risks to the health gases, machines and and safety of artists. This electrical hazards are the guide provides an overview most common health and of some of the most safety risks associated with common risks associated the visual arts. with painting, drawing, It is in the artists’ best photography, ceramics, interest to be informed of lithography, and sculpture. the toxic hazards of their All effort has been made to medium and how to use keep this document up to their materials safely. date with new non-toxic products and innovations as well as rediscovered traditional practices. TOXIC EFFECTS The health hazards associated with painting and drawing have been known since Ramazzini described them in his book, De Morbis Artificum Diatriba, in 1713. Working safely can involve changes in how you select your art materials, how you handle and dispose of them. Jay DeFeo, died at the age of sixty due, in large part, to her exposure to lead paints. Many artists of her day did not know what was in the materials they were using. In fact, manufacturers were not required to disclose that information until, for the first time in 1984, California legislation was introduced forcing manufactures to reveal the health hazards within their products. Jay DeFeo working on the painting, The Rose. -

THE FUTURE DIRECTIONS of TANNING* by ANTHONY D
7 QUO VADIT CHROMIUM? THE FUTURE DIRECTIONS OF TANNING* by ANTHONY D. COVINGTON* British School of Leather Technology The University of Northampton BOUGHTON GREEN ROAD, NORTHAMPTON NN2 7AL, UK ABSTRACT La pregunta expuesta es: donde nos llevará este nuevo concepto en términos del futuro de la curtición? From leather science, a deeper understanding of the La respuesta es: a un desplazamiento paradigmático. theoretical basis of the tanning mechanism has unified the inorganic and organic chemistries of Curtición al cromo permanecerá con nosotros, pero leather making processes. The 'link-lock' mechanism en un futuro se visualizará muy diferentemente provides an explanation of present reactions and a su tecnología por hoy percibida. Opciones para la tool for future developments. transformación del proceso ya sí se pueden proponer. Es importante en este ejercicio cuestionar detalladamente The question now posed is: where does this new los aspectos convencionalmente aceptados de thinking take us, in terms of the future of tanning? esta reacción. The answer is: into a paradigm shift. Tecnologías alternativas de curtición en la era Chrome tanning will remain with us, but in the moderna pueden ahora ser desarrolladas, para future it can be viewed very differently from its producir cueros altamente estables hidrotérmicamente. currently perceived technology. Options for Aquí la teoría eslabón-cerrojo ha demostrado que tales transforming the process may now be proposed. resultados no son obtenibles con un solo reactivo, por lo Important in this exercise is to question closely the menos dos componentes de curtido son requeridos. conventionally accepted features of the reaction. Más aun, la curtición podrá ser más acertadamente formulada para que encaje con la química (o bioquímica) Alternative technologies for tanning in the modern de la reacción según su rendimiento y los requerimientos era can now be developed, to make high hydrothermal del impacto ambiental del cuero. -

Potassium Dichromate CAS Number: 7778-50-9 EC Number: 231-906-6
21.5.2010 COMMENTS AND RESPONSE TO COMMENTS ON ANNEX XV SVHC: PROPOSAL AND JUSTIFICATION Substance name: Potassium dichromate CAS number: 7778-50-9 EC number: 231-906-6 Reason of the submission of the Annex XV: CMR Disclaimer: The European Chemicals Agency is not responsible for the content of this document. The Response to Comments table has been prepared by the competent authority of the Member State preparing the proposal for identification of a Substance of Very High Concern. The comments were received during the public consultation of the Annex XV dossier. General comments Date Submitted by (name, Comment Response Organisation/MSCA) 20100323 CLEAPSS, UK (on behalf of an CLEAPSS is an advisory service providing support in No answer expected. organisation) science and technology for a consortium of local authorities and their schools including establishments for pupils with special needs. Independent schools, post-16 colleges, teacher training establishments, curriculum developers and others can apply for associate membership. We offer help from nursery education through to A-level studies or equivalent. Our services cover health & safety, risk assessment, sources and use of chemicals, living organisms and equipment. CLEAPSS also provides advice on technicians & their jobs, as well as the design of laboratories and facilities & fittings for D&T and science rooms. 20100324 Germany, company (on behalf of an We reject the ban of Potassium Dichromate in general, Thank for your comment. organisation) because the substance is used in a low concentration (< 1%) in an almost closed system of cuvette tests 20100408 Draeger Safety AG & Co. KGaA, comments k2Cr2O7R1.doc Thank you for this information.