Massachusetts Chemical Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

Hexavalent Chromium Hexavalent
Toxic Heavy Metals Series Toxic Heavy Metals Hexavalent ChromiumHexavalent This document will familiarize the reader with the toxic metal Hexavalent Chromium, OSHA standards, and methods for prevention and decontamination. Hygenall Corporation 6275 University Drive, STE 37-390 Huntsville, AL 35806 256.781.4486 [email protected] Toxic Metal Series Hexavalent Chromium Contents Introduction . 3 Worker Exposure and Health Consequences . 4 OSHA’s Hexavalent Chromium Standards . 4 Exposure Limits . 4 Exposure Monitoring and Determinations . 4 Scheduled Monitoring Option . 5 Regulated Areas . 6 Control Measures . 7 Requirements for Protective Work Clothing and Equipment . 8 Hygiene Areas and Practices . 9 Housekeeping . 10 Recommended Cleaning and Decontamination Products…11 Medical Surveillance . 12 Worker Training and Communication . 13 Recordkeeping . 14 Effective Dates . 14 Additional Information . 15 References . 16 2 | P a g e Hexavalent Chromium Toxic Metal Series INTRODUCTION This document is intended to give readers an overview of the provisions and requirements of the OSHA Hexavalent Chromium standards for general industry (29 CFR 1910.1026), shipyards (29 CFR 1915.1026), and construction (29 CFR 1926.1126). Hexavalent chromium (Cr(VI)) is a toxic form of the element chromium. Hexavalent chromium is rarely found in nature and is generally man-made. Cr(VI) is widely used in pigments, metal finishing electroplating), wood preservatives and fungicides, and in chemical synthesis as an ingredient and catalyst. Table 1 lists some selected Cr(VI) compounds with their synonyms and common uses. Hexavalent chromium may also be present in fumes generated during the production or welding of chrome alloys. Chromium metal is often alloyed with other metals or plated on metal and plastic substrates to improve corrosion resistance and provide protective coatings. -

Chromium Compounds Hazard Summary
Chromium Compounds Hazard Summary Chromium occurs in the environment primarily in two valence states, trivalent chromium (Cr III) and hexavalent chromium (Cr VI). Exposure may occur from natural or industrial sources of chromium. Chromium III is much less toxic than chromium (VI). The respiratory tract is also the major target organ for chromium (III) toxicity, similar to chromium (VI). Chromium (III) is an essential element in humans. The body can detoxify some amount of chromium (VI) to chromium (III). The respiratory tract is the major target organ for chromium (VI) toxicity, for acute (short-term) and chronic (long-term) inhalation exposures. Shortness of breath, coughing, and wheezing were reported from a case of acute exposure to chromium (VI), while perforations and ulcerations of the septum, bronchitis, decreased pulmonary function, pneumonia, and other respiratory effects have been noted from chronic exposure. Human studies have clearly established that inhaled chromium (VI) is a human carcinogen, resulting in an increased risk of lung cancer. Animal studies have shown chromium (VI) to cause lung tumors via inhalation exposure. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (7), which contains information on inhalation chronic toxicity and the RfC and oral chronic toxicity and the RfD, and the carcinogenic effects of chromium including the unit cancer risk for inhalation exposure, EPA's Toxicological Review of Trivalent Chromium and Toxicological Review of Hexavalent Chromium (3), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Chromium. (1) Uses The metal chromium is used mainly for making steel and other alloys. -

Coast Guard, DHS Pt. 150, App. I
Coast Guard, DHS Pt. 150, App. I APPENDIX I TO PART 150—EXCEPTIONS Member of reactive group Compatible with TO THE CHART Propylene glycol (20) (a) The binary combinations listed below Oleum (0) ............................... Hexane (31) have been tested as prescribed in Appendix Dichloromethane (36) III and found not to be dangerously reactive. Perchloroethylene (36) These combinations are exceptions to the 1,2-Propylene glycol (20) ...... Diethylenetriamine (7) Compatibility Chart (Figure 1) and may be Polyethylene polyamines (7) Triethylenetetramine (7) stowed in adjacent tanks. Sodium dichromate, 70% (0) Methyl alcohol (20) Member of reactive group Compatible with Sodium hydrosulfide solution Methyl alcohol (20) (5). Acetone (18) .......................... Diethylenetriamine (7) Iso-Propyl alcohol (20) Acetone cyanohydrin (0) ....... Acetic acid (4) Sulfuric acid (2) ..................... Coconut oil (34) Acrylonitrile (15) ..................... Triethanolamine (8) Coconut oil acid (34) Palm oil (34) 1,3-Butylene glycol (20) ......... Morpholine (7) Tallow (34) 1,4-Butylene glycol (20) ......... Ethylamine (7) Sulfuric acid, 98% or less (2) Choice white grease tallow Triethanolamine (8) (34) gamma-Butyrolactone (0) ...... N-Methyl-2-pyrrolidone (9) Caustic potash, 50% or less Isobutyl alcohol (20) (b) The binary combinations listed below (5). Ethyl alcohol (20) have been determined to be dangerously re- Ethylene glycol (20) active, based on either data obtained in the Isopropyl alcohol (20) Methyl alcohol (20) literature or on laboratory testing which has iso-Octyl alcohol (20) been carried out in accordance with proce- Caustic soda, 50% or less (5) Butyl alcohol (20) dures prescribed in Appendix III. These com- tert-Butyl alcohol, Methanol binations are exceptions to the Compat- mixtures ibility Chart (Figure 1) and may not be Decyl alcohol (20) stowed in adjacent tanks. -

How to Handle Cryogenic Liquids
Photographic Processing Hazards, by Michael McCann, Ph.D., CIH • Black-and-White Photographic Processing o Mixing Photochemicals o Developing Baths o Stop Baths and Fixers o Intensifiers and Reducers o Toners o Other Hazards • Color Processing o Developing Baths o Bleaching, Fixing, and Other Steps Black-and-White Photographic Processing A wide variety of chemicals are used in black and white photographic processing. Film developing is usually done in closed canisters. Print processing uses tray processing, with successive developing baths, stop baths, fixing baths, and rinse steps. Other treatments include use of hardeners, intensifiers, reducers, toners, and hypo eliminators. Mixing Photochemicals Photochemicals can be bought in liquid form, which only need diluting, or powder form, which need dissolving and diluting. Hazards 1. Developer solutions and powders are often highly alkaline, and glacial acetic acid, used in making the stop bath, is also corrosive by skin contact, inhalation and ingestion. 2. Developer powders are highly toxic by inhalation, and moderately toxic by skin contact, due to the alkali and developers themselves (see Developing Baths below). Precautions 1. Use liquid chemistry whenever possible, rather than mixing developing powders. Pregnant women, in particular, should not be exposed to powdered developer. 2. When mixing powdered developers, use a glove box (a cardboard box with glass or plexiglas top, and two holes in the sides for hands and arms), local exhaust ventilation, or wear a NIOSH-approved toxic dust respirator. 3. Wear gloves, goggles and protective apron when mixing concentrated photochemicals. Always add any acid to water, never the reverse. 4. In case of skin contact, rinse with lots of water. -

Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium
Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium Glyn R. Fox Environmental Management Branch Environmental Protection Division September 24, 2007 Victoria, British Columbia Table of Contents Page 1. Introduction ……………………………………………………………………… 4 2. Details Related to Derivation of Proposed 2007 Draft Matrix Soil Standards 2.1 Derivation of Human Health Protection Standards – Intake of contaminated soil ………………………………………………………….. 7 2.2 Derivation of Human health Protection Standards – Groundwater used for drinking water ……………………………….…………….......... 7 2.3 Derivation of Environmental Protection Standards – Toxicity to soil invertebrates and plants ……………………………………….......... 9 2.4 Derivation of Environmental Protection Standards – Livestock ingesting soil and fodder ………………………………………………… 9 2.5 Derivation of Environmental Protection Standards – Major microbial function impairment ………………………………….……… 10 2.6 Derivation of Environmental Protection Standards – Groundwater flow to surface water used by aquatic life ..…………………………… 10 2.7 Derivation of Environmental Protection Standards – Groundwater used for livestock watering ……………………………………………… 11 2.8 Derivation of Environmental Protection Standards – Groundwater used for irrigation ………………………………………………………… 12 2.9 CSST “Background” Adjustment ……………………..……………… 12 2.10 Application of CSST Rounding-off Rule ……………………………… 12 3. References …….…………………………………………………………………… 13 2 Table of Contents (continued) Page 4. Exhibits Exhibit 1. Proposed 2007 Draft Matrix Soil Standards for Barium ……..… 19 5. Tables -

Beneficial Reuse and Waste Minimization of Hexavalent Chrome
Hexavalent Chromium Treatment Technologies Research and Development Office Science and Technology Program Final Report ST-2018-9085-01 U.S. Department of the Interior Bureau of Reclamation Research and Development Office 10/1/2018 Mission Statements Protecting America's Great Outdoors and Powering Our Future The Department of the Interior protects and manages the Nation's natural resources and cultural heritage; provides scientific and other information about those resources; and honors its trust responsibilities or special commitments to American Indians, Alaska Natives, and affiliated island communities. The following form is a Standard form 298, Report Documentation Page. This report was sponsored by the Bureau of Reclamations Research and Development office. For more detailed information about this Report documentation page please contact Miguel Arias-Paic at 303-445-2131. THIS TEXT WILL BE INVISIBLE. IT IS FOR 508 COMPLIANCE OF THE NEXT PAGE. Disclaimer Information in this report may not be used for advertising or promotional purposes. The data and findings should not be construed as an endorsement of any product or firm by the Bureau of Reclamation, Department of Interior, or Federal Government. The products evaluated in the report were evaluated for purposes specific to the Bureau of Reclamation mission. Reclamation gives no warranties or guarantees, expressed or implied, for the products evaluated in this report, including merchantability or fitness for a particular purpose. Form Approved REPORT DOCUMENTATION PAGE OMB No. 0704-0188 T1. REPORT DATE: T2. REPORT TYPE: T3. DATES COVERED SEPTEMBER 2018 RESEARCH T4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER Hexavalent Chromium Treatment Technologies Z1759, X9085, FA280 5b. -

Jason Lotter, MS, CIH
Jason Lotter, MS, CIH Current Position Summary of Experience Supervising Health Mr. Jason Lotter is a Supervising Health Scientist with Cardno ChemRisk in Chicago. His Scientist principal areas of training and expertise include industrial hygiene, exposure and risk assessment, and occupational safety. He has been involved in researching, measuring, Discipline Areas and reconstructing exposures, and assessing risks to consumers, residents, and workers, > Industrial Hygiene from a variety of chemicals including formaldehyde, silica, diacetyl, coal dust, lead, > Exposure particulate matter, hexavalent chromium, asbestos, and metals. Mr. Lotter completed his Assessment master’s degree in Public Health Science, with an emphasis in industrial hygiene, from > Risk Assessment the University of Illinois at Chicago. For his master’s thesis, he evaluated groundwater > Occupational Safety arsenic in Guatemala and conducted a risk assessment related to arsenic exposure for local residents. Years' Experience 7 Years At ChemRisk, Mr. Lotter has conducted independent research on various topics and chemicals, and has presented his research at scientific conferences and published in the Joined Cardno peer-reviewed literature. He has also conducted indoor air modeling for formaldehyde, 2013 provided industrial hygiene and occupational safety support during refinery turnarounds, and has assisted in the completion of various exposure simulation studies. Education > MS, Public Health Mr. Lotter currently co-leads the Cardno ChemRisk Cleaning and Disinfectant Product Science, University initiative, focusing on peracetic acid and other antimicrobial chemicals. He is a member of of Illinois at Chicago, the American Industrial Hygiene Association (AIHA) and currently serves on the Board of 2012 Directors of the Chicago Local Section of AIHA. He has been as a Certified Industrial > BS, Civil Hygienist (CIH) since 2016. -
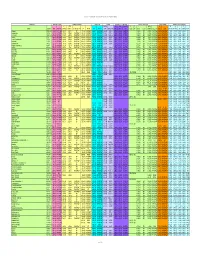
Chemical-Specific Parameters Supporting Table May 2016 Analyte
Regional Screening Level (RSL) Chemical-specific Parameters Supporting Table May 2016 Contaminant Molecular Weight Volatility Parameters Melting Point Density Diffusivity in Air and Water Partition Coefficients Water Solubility Tapwater Dermal Parameters H` (atm- Density Dia Diw Dia and Diw Kd Kd Koc log Kow S B τevent t* Kp 3 3 2 2 Analyte CAS No. MW MW Ref (unitless) m /mole) H` and HLC Ref VP VP Ref MP MP Ref (g/cm ) Density Ref (cm /s) (cm /s) Ref (L/kg) Ref (L/kg) Koc Ref (unitless) log Kow Ref (mg/L) S Ref (unitless) (hr/event) (hr) (cm/hr) K Ref Acephate 30560-19-1 1.8E+02 PHYSPRO 2.0E-11 5.0E-13 EPI 1.7E-06 PHYSPROP 8.8E+01 PHYSPROP 1.4E+00 CRC89 3.7E-02 8.0E-06 WATER9 1.0E+01 EPI -8.5E-01 PHYSPRO 8.2E+05 PHYSPROP 2.1E-04 1.1E+00 2.7E+00 4.0E-05 EPI Acetaldehyde 75-07-0 4.4E+01 PHYSPRO 2.7E-03 6.7E-05 PHYSPROP 9.0E+02 PHYSPROP -1.2E+02 PHYSPROP 7.8E-01 CRC89 1.3E-01 1.4E-05 WATER9 1.0E+00 EPI -3.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.3E-03 1.9E-01 4.5E-01 5.3E-04 EPI Acetochlor 34256-82-1 2.7E+02 PHYSPRO 9.1E-07 2.2E-08 PHYSPROP 2.8E-05 PHYSPROP 1.1E+01 PubChem 1.1E+00 PubChem 2.2E-02 5.6E-06 WATER9 3.0E+02 EPI 3.0E+00 PHYSPRO 2.2E+02 PHYSPROP 3.1E-02 3.4E+00 8.2E+00 5.0E-03 EPI Acetone 67-64-1 5.8E+01 PHYSPRO 1.4E-03 3.5E-05 PHYSPROP 2.3E+02 PHYSPROP -9.5E+01 PHYSPROP 7.8E-01 CRC89 1.1E-01 1.2E-05 WATER9 2.4E+00 EPI -2.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.5E-03 2.2E-01 5.3E-01 5.1E-04 EPI Acetone Cyanohydrin 75-86-5 8.5E+01 PHYSPRO 8.1E-08 2.0E-09 PHYSPROP 3.4E-01 PHYSPROP -1.9E+01 PHYSPROP 9.3E-01 CRC89 8.6E-02 1.0E-05 WATER9 1.0E+00 -

The Influence of Heavy Metals and Organic Matter on Hexavalent
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza 289 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 47, 2016 The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Angelo Chianese, Luca Di Palma, Elisabetta Petrucci, Marco Stoller Copyright © 2016, AIDIC Servizi S.r.l., ISBN 978-88-95608-38-9; ISSN 2283-9216 DOI: 10.3303/CET1647049 The Influence of Heavy Metals and Organic Matter on Hexavalent Chromium Reduction by Nano Zero Valent Iron in Soil Mouhamadou Thierno Gueyea, Luca Di Palmab, Gunel Allahverdeyevac, Irene b b b b Bavasso , Elisabetta Petrucci , Marco Stoller , Giorgio Vilardi a Université Cheikh Anta Diop de Dakar (UCAD), Dakar Fann, Senegal b Sapienza Università di Roma, Dipartimento di Ingegneria Chimica Materiali Ambiente, via Eudossiana 18, 00184 Roma c Baku State University, 23 Khalilov St., Baku, Azerbaijan During the last decades great attention has been payed at evaluating the feasibility of Cr(VI) reduction in soil 0 by nano zero valent iron (nZVI). An inhibitory effect on the Cr(VI) reduction by Fe nanoparticles is generally shown in the presence of high level of heavy metals and natural organic matter in soil. Heavy metals in the environment can react with nZVI by redox reactions, precipitation/dissolution reactions, and adsorption/desorption phenomena. As a result of the presence of metals as Ni, Pb, a decrease in the rate of Cr(VI) reduction was observed. Hence, in the present study, experimental tests of Cr(VI) reduction by nZVI in the presence of selected heavy metals, such as nickel and lead, and in the presence of high level of organic matter, are presented and discussed. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table.