CHROMIUM and Ehromium Eompounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wood Modified by Inorganic Salts: Mechanism and Properties
WOOD MODIFIED BY INORGANIC SALTS: MECHANISM AND PROPERTIES. I. WEATHERING RATE, WATER REPELLENCY, AND IIIMENSIONAL STABILITY OF WOOD MODIFIEID WITH CHROMIUM (111) NITRATE VERSUS CHROMIC ACID' R. S. Willianzs and W. C. Feisl Research Chemists Forest Products Lab~ratory,~Forest Service U.S. Department of Agriculture Madison, WI 53705 (Received October 1983) ABSTRACT Chromic acid treatment of wood improves surface characteristics. A simple dip or brush application of 5% aqueous chromic acid to a wood surface stops extractive staining, improves dimensional stability, retards weathering of unfinished wood, and prolongs the life of finishes. A better understanding of how chromic acid effects these improvements may facilitate the development of even better treatments. The improvements may be due to a combination offactors: oxidation ofwood by hexavalent chromium compounds, formation of coordination coml~lexeswith trivalent chromium, and the presence of insoluble chromium compounds at the surface. Most trivalent chromium compounds do not become fixed in wood (i.e., do not form water-insolublf: wood-chemical complexes). However, chromium (111) nitrate treatment of western redcedar (Thuja plicata), southern pine (Pinus sp.), and ponderosa pine (Pinus ponderosa) produced modified wood with properties similar to chromic acid-treated wood. Evaluation of the chromium (111) nitrate- and chromic acid-modified wood by leaching, Xenon arc- accelerated weathering, and swellometer experiments clearly demonstrated similar fixation, erosion, and water-repellent properties. Based on the results from chromium (111) nitrate-treated wood, fixation through coordination of trivalent chromium with wood hydroxyls an(5 formation of insoluble chromium appear to be the critical factors in improving the performance of the treated wood. -

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

Chromium Compounds Hazard Summary
Chromium Compounds Hazard Summary Chromium occurs in the environment primarily in two valence states, trivalent chromium (Cr III) and hexavalent chromium (Cr VI). Exposure may occur from natural or industrial sources of chromium. Chromium III is much less toxic than chromium (VI). The respiratory tract is also the major target organ for chromium (III) toxicity, similar to chromium (VI). Chromium (III) is an essential element in humans. The body can detoxify some amount of chromium (VI) to chromium (III). The respiratory tract is the major target organ for chromium (VI) toxicity, for acute (short-term) and chronic (long-term) inhalation exposures. Shortness of breath, coughing, and wheezing were reported from a case of acute exposure to chromium (VI), while perforations and ulcerations of the septum, bronchitis, decreased pulmonary function, pneumonia, and other respiratory effects have been noted from chronic exposure. Human studies have clearly established that inhaled chromium (VI) is a human carcinogen, resulting in an increased risk of lung cancer. Animal studies have shown chromium (VI) to cause lung tumors via inhalation exposure. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (7), which contains information on inhalation chronic toxicity and the RfC and oral chronic toxicity and the RfD, and the carcinogenic effects of chromium including the unit cancer risk for inhalation exposure, EPA's Toxicological Review of Trivalent Chromium and Toxicological Review of Hexavalent Chromium (3), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Chromium. (1) Uses The metal chromium is used mainly for making steel and other alloys. -

Health Hazard Evaluation Report 77-63-449
\ U.S. DEPARTMENT OF HE1\LTH, EDUCATI0f1, AND WELFARE CENTER FOR DISEASE COllTROL I NATIONAL INSTITUTE FOR OCCUPATIOfU\L SAFETY AND HEALTH i CINCINNATI, OHIO 45226 HEALTH HAZARD EVALUATIOf~ DETERMINATION l REPORT NO. 77-63-449 McDONNELL AIRCRAFT COMPANY ST. LOUIS, MISSOURI DECEMBER 1977 I. TOXICITY DETERMINATION A health hazard evaluation was conducted by the National Institute for Occupational Safety and Health on July 11-14, 1977, at the McDonnell Aircraft Company in St. Louis, Missouri. Based on the medical evaluation of employees in Department 151 and the industrial hygiene survey, it is determined that employees were not exposed to toxic concentrations of contaminants during this evaluatton. Potential contaminants studied included: iron oxide, nickel, chromium, sulfuric acid, sodium hydroxide, hydrochloric acid, hydrofluoric acid, nitric acid, hydrogen sulfide, sulfur dioxide, nitrogen dioxide, nitric oxide, toluene, xylene, benzene, refined petroleum solvents, tetrachloroethylene, acetone, and styrene. It is our opinion that workers in the masking operations may occasionally be exposed to concentrations of organic solvents, and workers involved in the etching operations may occasionally be exposed to concentrations of acids and/or associated emissions such as nitric acid, nitrogen dioxide and nitric oxide which have produced medical symptoms among the employees. These substances are known to produce nose, throat and skin irritation, dizziness and headaches - symptoms which were reported by workers in private interviews during the survey and are important indications of excessive exposure. From this limited evaluation, the authors feel that no definitive statement can be made concerning the workers in Department 151 having an increased incidence of deaths which could be directly attributed to occupational exposure. -

Chromic Acid Chromium Trioxide Flake
CHROMIC ACID CHROMIUM TRIOXIDE FLAKE PROPERTIES: Chemical Formula: Stability in Air: CrO3 Deliquesces at humidity above 30% CAS Number: 1333-82-0 Solubility in Water: Highly soluble: 62.5% w/w at 20 ºC ( 68 ºF) 67.5% w/w at 100 ºC (212 ºF) Molecular Weight: 99.99 Bulk Density Loose 75 - 82 lbs. per cubic foot Appearance: Dark Red Flakes Heat of Solution Exothermic (24.7 cal./g.; 44.5 BTU/lb.) ThroughApplied Innovation Specific Gravity: 2.7 at 20 °C (68 °F) Behavior on Heating Melts at 197 ºC (387 ºF) - starts to decompose to intermediate chromium oxides at slightly higher temperatures. Chemical Characteristics: Performance d Chromic acid is a strong acid and oxidizing agent. When neutralized with alkalis, chromic acid forms dichromate or chromate compounds. In oxidation reactions, the chromium atom is reduced from the hexavalent to trivalent Enhance state. continued… www.elementis.com CHROMIC ACID CHROMIUM TRIOXIDE FLAKE TYPICAL ANALYSIS: * Specification Typical Analysis CrO3 99.85 % Min. 99.9 % Sulfate as SO4 0.10 % Max. 0.07 % Chloride as Cl 0.005 % Max. <0.005 % Insoluble Matter 0.005 % Max. 0.002 % Sodium as Na2O 0.020 % Max. 0.014 % pH of 1% Aqueous Solution NA 1.0 Meets GSA Commercial Item Description A-A-55827D, 20 April 2015 GENERAL APPLICATIONS: ThroughApplied Innovation The major uses of chromic acid are in wood preservation, metal finishing and plating. Secondary applications include catalyst manufacture and as an oxidizing agent. Chromic acid is used to produce salt-free CCA type wood preservatives. These preservatives are combinations of chromium, copper and arsenic. -
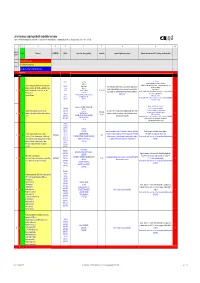
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Chromic Acid, Nitric Acid, Hydroxyl Compounds, Ethylene Glycol
Acetic acid - Chromic acid, nitric acid, hydroxyl compounds, ethylene glycol, perchloric acid, peroxides, permanganates, ammonium nitrate Acetic anhydride - Hydroxyl-containing compounds such as ethylene glycol, perchloric acid Acetone - Concentrated nitric and sulfuric acid mixtures Acetaldehyde - Acetic acid, acetic anhydride Acetylene - Chlorine, bromine, copper, fluorine, silver, mercury Acrolein - Ammonia(aqueous), any alkali or amine, strong oxidizing agents Alkali and alkaline earth metals (such as powdered aluminum or magnesium, calcium, lithium, sodium, potassium) - Water, carbon tetrachloride or other chlorinated hydrocarbons, carbon dioxide, halogens. Aluminum metal - Ammonium nitrate, antimony trichloride, bromate Ammonia (anhydrous) - Mercury (in manometers, for example), chlorine, calcium hypochlorite, iodine, bromine, hydrofluoric acid (anhydrous) Ammonium nitrate - Acids, powdered metals, flammable liquids, chlorates, nitrites, sulfur, finely divided organic combustible materials Aniline - Nitric acid, hydrogen peroxide, strong acids, oxidizers Arsenic materials - Any reducing agent Azides – Acids Bromine - Ammonia, acetylene, butadiene, butane, methane, propane (or other petroleum gases), hydrogen, sodium carbide, benzene, finely divided metals, turpentine Calcium oxide – Water Carbon (activated) - Calcium hypochlorite, all oxidizing agents Carbon tetrachloride – Sodium Chlorates - Ammonium salts, acids, powdered metals, sulfur, finely divided organic or combustible materials Chromic acid and chromium trioxide - Acetic -

Chromium Trioxide, Sodium Chromate, Sodium Dichromate, Ammonium Dichromate and Potassium Dichromate
Institute for Health and Consumer Protection European Chemicals Bureau I-21020 Ispra (VA) Italy CHROMIUM TRIOXIDE, SODIUM CHROMATE, SODIUM DICHROMATE, AMMONIUM DICHROMATE AND POTASSIUM DICHROMATE CAS No: 1333-82-0, 7775-11-3, 10588-01-9, 7789-09-5 and 7778-50-9 EINECS No: 215-607-8, 231-889-5, 234-190-3, 232-143-1 and 231-906-6 Summary Risk Assessment Report 2005 Special Publication I.05.16 CHROMIUM TRIOXIDE, SODIUM CHROMATE, SODIUM DICHROMATE, AMMONIUM DICHROMATE AND POTASSIUM DICHROMATE CAS No: 13333-82-0, 7775-11-3, 10588-01-9, 7789-09-5 and 7778-50-9 EINECS No: 215-607-8, 231-889-5, 234-190-3, 232-143-1 and 231-906-6 SUMMARY RISK ASSESSMENT REPORT Final report,2005 United Kingdom This document has been prepared by the UK rapporteur on behalf of the European Union. The scientific work on the environmental sections was carried out by the Building Research Establishment (BRE), under contract to the rapporteur. The distribution of this draft risk assessment report is the responsibility of the rapporteur. Anyone wishing to cite, quote or copy this report must obtain the permission of the rapporteur beforehand. Contact point: Rapporteur: United Kingdom Contact - human health: Health & Safety Executive Industrial Chemicals Unit Magdalen House Stanley Precinct Bootle, Merseyside L20 3QZ [email protected] Tel: (44) 0151 951 4564 Fax: (44) 0151 951 3308 Contact - environment: Environment Agency Chemicals Assessment Section Ecotoxicology & Hazardous Substances National Centre Isis House, Howbery Park Wallingford Oxfordshire OX10 8BD Tel: (44) 01491 828 559 Fax: (44) 01491 828 556 Date of Last Literature Search: 2000 Review of report by MS Technical Experts finalised: 2002 Final report: 2005 © European Communities, 2005 PREFACE This report provides a summary, with conclusions, of the risk assessment report of the substances chromium trioxide, sodium chromate, sodium dichromate, ammonium dichromate and potassium dichromate that have been prepared by the United Kingdom in the context of Council Regulation (EEC) No. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 3/9/2017 Revision Date: 3/9/2017 Revision Number: G1 1. IDENTIFICATION Product identifier Product code: C1255 Product Name: CHROMIUM OXIDE, GREEN, POWDER, PURIFIED Other means of identification Synonyms: Anhydride chromique [French] C.I. 77288 C.I. Pigment Green 17 Chrome Green Chrome Ocher Chrome ochre Chrome oxide Chromic Acid Green Chromic oxide Chromium Oxide Green Chromium oxide Chromium sesquioxide Chromium trioxide Chromium(3+) oxide Chromium(3+) trioxide Chromium(III) oxide Chromium(III) oxide (2:3) Dichromium trioxide Green Chrome Oxide Green Oxide of Chromium Green chromic oxide Green chromium oxide Green cinnabar Oxide of chromium CAS #: 1308-38-9 RTECS # GB6475000 CI#: 77288 Recommended use of the chemical and restrictions on use Recommended use: Pigment. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000. Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) Product code: C1255 Product name: CHROMIUM OXIDE, 1 / 11 GREEN, POWDER, PURIFIED 2. HAZARDS IDENTIFICATION Classification This chemical is not considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Not a dangerous substance or mixture according to the Globally Harmonized System (GHS) Label elements Not classified Hazards not otherwise classified (HNOC) Not Applicable Other hazards Not available 3. COMPOSITION/INFORMATION ON INGREDIENTS Components CAS-No. Weight % Chromium Oxide 1308-38-9 100 4. FIRST AID MEASURES First aid measures General Advice: National Capital Poison Center in the United States can provide assistance if you have a poison emergency and need to talk to a poison specialist. -

Sd0000029 Evaluation of Chromfte Ore and The
SD0000029 EVALUATION OF CHROMFTE ORE AND THE OPTIMUM METHODS FOR INDUSTRIAL EXTRACTION OF CHROMIUM A TI1HSIS SUBMITTED BY Bakheit Mustafa Mohamed Salih IN CANDIDATURE FOR TIU:DI:GRFJ:OI MASTER OF SC1HNCT DEPARTMENT OF CHEMISTRY FACULTY OF SCIENCE (P.O.BOX 321) OCTOBER 1999 UNIVERSITY OF KHARTOUM 31/ 28 ABSTRACT Samples of chromite ore, collected from Gam and Cheikay mining area (Ingessana Hills) in east Sudan, were analysed to assess the chromium content. Methods for extraction and analysis of chromium metal were developed and established. Analysis were carried out using atomic absorption spectroscopy (AAS) to estimate the contents of chromium, iron, calcium, and magnesium. X-ray fluorescence (XRF) was used to evaluate the levels of chromium, iron, and calcium in the ore. Volumetric analysis was performed to assess chromium and iron, whilst gravimetric analysis was employed to measure the amounts of calcium, magnesium, aluminum and silicon present in the ore. The data was chemically and statistically analyzed to compare the results obtained by the given analytical methods. The results are in good agreement except iron oxide, which displayed a significantly different value when measured by x-ray fluorescence. The data obtained exhibited similarity in almost all cases, when compared with local and global researches, reports, and literature. The study has revealed the average contents of Cr2O3, FeO, CaO, MgO, A12O3, and SiO2 as 40.66. 11.96, 11.94. 0.36. 16.94, 11.45% respectively. MnO and NiO were detected in trace amounts, the corresponding levels in the ore being 72 and 27 ppm. The average chromium content in extracted potassium dichromate measured by using AAS, XRF, and volumetric methods was found to be 31.7%. -

Potassium Dichromate CAS Number: 7778-50-9 EC Number: 231-906-6
21.5.2010 COMMENTS AND RESPONSE TO COMMENTS ON ANNEX XV SVHC: PROPOSAL AND JUSTIFICATION Substance name: Potassium dichromate CAS number: 7778-50-9 EC number: 231-906-6 Reason of the submission of the Annex XV: CMR Disclaimer: The European Chemicals Agency is not responsible for the content of this document. The Response to Comments table has been prepared by the competent authority of the Member State preparing the proposal for identification of a Substance of Very High Concern. The comments were received during the public consultation of the Annex XV dossier. General comments Date Submitted by (name, Comment Response Organisation/MSCA) 20100323 CLEAPSS, UK (on behalf of an CLEAPSS is an advisory service providing support in No answer expected. organisation) science and technology for a consortium of local authorities and their schools including establishments for pupils with special needs. Independent schools, post-16 colleges, teacher training establishments, curriculum developers and others can apply for associate membership. We offer help from nursery education through to A-level studies or equivalent. Our services cover health & safety, risk assessment, sources and use of chemicals, living organisms and equipment. CLEAPSS also provides advice on technicians & their jobs, as well as the design of laboratories and facilities & fittings for D&T and science rooms. 20100324 Germany, company (on behalf of an We reject the ban of Potassium Dichromate in general, Thank for your comment. organisation) because the substance is used in a low concentration (< 1%) in an almost closed system of cuvette tests 20100408 Draeger Safety AG & Co. KGaA, comments k2Cr2O7R1.doc Thank you for this information. -

Rpt POL-TOXIC AIR POLLUTANTS 98 BY
SWCAA TOXIC AIR POLLUTANTS '98 by CAS ASIL TAP SQER CAS No HAP POLLUTANT NAME HAP CAT 24hr ug/m3 Ann ug/m3 Class lbs/yr lbs/hr none17 BN 1750 0.20 ALUMINUM compounds none0.00023 AY None None ARSENIC compounds (E649418) ARSENIC COMPOUNDS none0.12 AY 20 None BENZENE, TOLUENE, ETHYLBENZENE, XYLENES BENZENE none0.12 AY 20 None BTEX BENZENE none0.000083 AY None None CHROMIUM (VI) compounds CHROMIUM COMPOUN none0.000083 AY None None CHROMIUM compounds (E649962) CHROMIUM COMPOUN none0.0016 AY 0.5 None COKE OVEN COMPOUNDS (E649830) - CAA 112B COKE OVEN EMISSIONS none3.3 BN 175 0.02 COPPER compounds none0.67 BN 175 0.02 COTTON DUST (raw) none17 BY 1,750 0.20 CYANIDE compounds CYANIDE COMPOUNDS none33 BN 5,250 0.60 FIBROUS GLASS DUST none33 BY 5,250 0.60 FINE MINERAL FIBERS FINE MINERAL FIBERS none8.3 BN 175 0.20 FLUORIDES, as F, containing fluoride, NOS none0.00000003 AY None None FURANS, NITRO- DIOXINS/FURANS none5900 BY 43,748 5.0 HEXANE, other isomers none3.3 BN 175 0.02 IRON SALTS, soluble as Fe none00 AN None None ISOPROPYL OILS none0.5 AY None None LEAD compounds (E650002) LEAD COMPOUNDS none0.4 BY 175 0.02 MANGANESE compounds (E650010) MANGANESE COMPOU none0.33 BY 175 0.02 MERCURY compounds (E650028) MERCURY COMPOUND none33 BY 5,250 0.60 MINERAL FIBERS ((fine), incl glass, glass wool, rock wool, slag w FINE MINERAL FIBERS none0.0021 AY 0.5 None NICKEL 59 (NY059280) NICKEL COMPOUNDS none0.0021 AY 0.5 None NICKEL compounds (E650036) NICKEL COMPOUNDS none0.00000003 AY None None NITROFURANS (nitrofurans furazolidone) DIOXINS/FURANS none0.0013