Colorimetric Studies on the Nature of Chromate Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHEMICAL STORAGE SEGREGATION GUIDELINES Incompatible Chemicals Should Always Be Handled and Stored So That They Do Not Accidentally Come in Contact with Each Other
Laboratory Safety Reminders January 2007 ♦ Mount Holyoke College – Environmental Health and Safety CHEMICAL STORAGE SEGREGATION GUIDELINES Incompatible chemicals should always be handled and stored so that they do not accidentally come in contact with each other. This list is not complete, nor are all compatibilities shown. These materials can react to produce excessive heat, harmful vapors, and/or other deadly reactions. Always know the hazards and incompatibilities of a chemical before using it. Chemicals Avoid Accidental Contact With Acetic acid Chromic acid, nitric acid, permanganates, peroxides Hydroxyl-containing compounds such as perchloric acid, Acetic anhydride ethylene glycol Concentrated nitric acid and sulfuric acid mixtures, peroxides (i.e. Acetone peracetic acid solution, hydrogen peroxide) Acetylene Chlorine, bromine, copper, silver, fluorine, mercury Alkali, alkaline earth and strongly electropositive metals (powered Carbon dioxide, carbon tetrachloride and other chlorinated aluminum, magnesium, sodium, hydrocarbons potassium) Mercury, chlorine, calcium hypochlorite, iodine, bromine, hydrogen Ammonia (anhydrous) fluoride Acids, metal powders, flammable liquids, chlorates, nitrates, sulfur, Ammonium nitrate finely divided organics, combustibles Aniline Nitric acid, hydrogen peroxide Arsenical compounds Any reducing agent Azides Acids Ammonia, acetylene, butadiene, butane, other petroleum gases, Bromine sodium carbide, turpentine, benzene, finely divided metals Calcium oxide Water Carbon activated Calcium hypochlorite, other -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Hazard Summary Identification Reason For
Common Name: CHROMIC ACID CAS Number: 7738-94-5 DOT Number: NA 1463 (Solid) RTK Substance number: 0429 UN 1755 (Solution) Date: September 1996 Revision: July 2002 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Chromic Acid can affect you when breathed in and by * Exposure to hazardous substances should be routinely passing through your skin. evaluated. This may include collecting personal and area * Chromic Acid should be handled as a CARCINOGEN-- air samples. You can obtain copies of sampling results WITH EXTREME CAUTION. from your employer. You have a legal right to this * Chromic Acid can cause reproductive damage. Handle information under OSHA 1910.1020. with extreme caution. * If you think you are experiencing any work-related health * Chromic Acid is a CORROSIVE CHEMICAL and problems, see a doctor trained to recognize occupational contact can severely irritate and burn the skin and eyes diseases. Take this Fact Sheet with you. with possible eye damage. * Breathing Chromic Acid can irritate the nose, throat and WORKPLACE EXPOSURE LIMITS lungs causing coughing, wheezing and/or shortness of The following exposure limits are for Chromic Acid breath. (measured as Chromium VI): * Chromic Acid can cause a sore and/or hole in the “bone” dividing the inner nose, sometimes with bleeding, OSHA: The legal airborne permissible exposure limit discharge or formation of a crust. (PEL) is 0.1 mg/m3, not to be exceeded at any * Chromic Acid may cause a skin allergy. If allergy time. develops, very low future exposure can cause itching and a skin rash. NIOSH: The recommended airborne exposure limit is * High exposure may affect the liver. -

Conductivity and Density of Chromic Acid Solutions
; RP198 CONDUCTIVITY AND DENSITY OF CHROMIC ACID SOLU- TIONS By H. R. Moore and W. Blum ABSTRACT The conductivity and density of chromic acid solutions were determined (<*r concentrations to molar up 10 (1,000 g/1 of Cr03 ). The density is practically a linear function of concentration. The conductivity increases to a maximum at about 5 molar and then decreases. CONTENTS Page I. Introduction 255 II. Preparation of solutions 256 1. Purification of chromic acid 256 2. Methods of analysis 257 (a) Preparation of samples 257 (6) Insoluble 257 (c) Sulphate 258 (d) Alkali salts 258 (e) Chromic acid 258 (/) Trivalent chromium 258 (1) Differential titration 258 (2) Direct precipitation 259 III. Conductivity measurements 259 1. Apparatus and method 259 2. Determination of cell constants 260 IV. Density measurements 260 V. Results obtained 261 VI. Conclusions 263 I. INTRODUCTION This study is a step in an investigation of the theory of chromium plating. The solutions used in this process have chromic acid as their principal constituent. In addition, they contain some other anion, such as the sulphate ion, which is introduced as sulphuric acid or as a sulphate. During operation there is always formed in the bath some trivalent chromium, commonly supposed to exist as "chromium chromates" of undefined composition. The ultimate purpose of this research is to determine the form and function of the sulphate and trivalent chromium in such solutions. The effects of these constituents upon the density and conductivity of chromic acid will they throw light on this problem. ; be measured to see whether First, however, it was necessary to determine the density and con- ductivity of pure chromic-acid solutions to serve as a basis of refer- ence. -
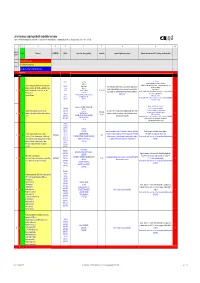
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Increte Stain-Crete
Version: 4.0 Revision Date: 08/23/2019 SAFETY DATA SHEET 1. Identification Material name: OB - STAIN-CRETE CHEM STAIN - 5 GL BLACK Material: CSCR G005 080 Recommended use and restriction on use Recommended use : Additive Restrictions on use: Not known. Manufacturer/Importer/Supplier/Distributor Information EUCLID CHEMICAL COMPANY 19218 REDWOOD ROAD CLEVELAND OH 44110 US Contact person : EH&S Department Telephone : 216-531-9222 Emergency telephone number : 1-800 -424 -9300 (US); 1 -613 -996 -6666 (Canada) 2. Hazard(s) identification Hazard Classification Health Hazards Acute toxicity (Oral) Category 4 Acute toxicity (Inhalation - dust and Category 2 mist) Skin Corrosion/Irritation Category 1A Serious Eye Damage/Eye Irritation Category 1 Respiratory sensitizer Category 1 Skin sensitizer Category 1 Germ Cell Mutagenicity Category 1B Carcinogenicity Category 1A Toxic to reproduction Category 1B Unknown toxicity - Health Acute toxicity, oral 0 % Acute toxicity, dermal 19.51 % Acute toxicity, inhalation, vapor 32.98 % Acute toxicity, inhalation, dust 19.51 % or mist Environmental Hazards Acute hazards to the aquatic Category 1 environment 1/17 000000013822 Version: 4.0 Revision Date: 08/23/2019 Unknown toxicity - Environment Acute hazards to the aquatic 73.06 % environment Chronic hazards to the aquatic 100 % environment Label Elements Hazard Symbol : Signal Word: Danger Hazard Statement: Fatal if inhaled. Harmful if swallowed. Causes severe skin burns and eye damage. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause an allergic skin reaction. May cause genetic defects. May cause cancer. May damage fertility or the unborn child. Very toxic to aquatic life. Precautionary Statements Prevention: Do not breathe dust/fume/gas/mist/vapors/spray. -

Carboxylic Acids
13 Carboxylic Acids The active ingredients in these two nonprescription pain relievers are derivatives of arylpropanoic acids. See Chemical Connections 13A, “From Willow Bark to Aspirin and Beyond.” Inset: A model of (S)-ibuprofen. (Charles D. Winters) KEY QUESTIONS 13.1 What Are Carboxylic Acids? HOW TO 13.2 How Are Carboxylic Acids Named? 13.1 How to Predict the Product of a Fischer 13.3 What Are the Physical Properties of Esterification Carboxylic Acids? 13.2 How to Predict the Product of a B-Decarboxylation 13.4 What Are the Acid–Base Properties of Reaction Carboxylic Acids? 13.5 How Are Carboxyl Groups Reduced? CHEMICAL CONNECTIONS 13.6 What Is Fischer Esterification? 13A From Willow Bark to Aspirin and Beyond 13.7 What Are Acid Chlorides? 13B Esters as Flavoring Agents 13.8 What Is Decarboxylation? 13C Ketone Bodies and Diabetes CARBOXYLIC ACIDS ARE another class of organic compounds containing the carbonyl group. Their occurrence in nature is widespread, and they are important components of foodstuffs such as vinegar, butter, and vegetable oils. The most important chemical property of carboxylic acids is their acidity. Furthermore, carboxylic acids form numerous important derivatives, including es- ters, amides, anhydrides, and acid halides. In this chapter, we study carboxylic acids themselves; in Chapters 14 and 15, we study their derivatives. 457 458 CHAPTER 13 Carboxylic Acids 13.1 What Are Carboxylic Acids? Carboxyl group A J COOH The functional group of a carboxylic acid is a carboxyl group, so named because it is made group. up of a carbonyl group and a hydroxyl group (Section 1.7D). -

Polypropylene Chemical Resistance Guide Chemical Listing & Ratings
Polypropylene Chemical Resistance Guide Chemical Listing & Ratings hmcpolymers.com POLYPROPYLENE CHEMICAL RESISTANCE About HMC Polymers HMC Polymers is focused on being PP chemical resistance Asia’s number ONE in PP. HMC Polymers’ polypropylene resins, like most HMC PP resins are appreciably affected Some reduction in tensile strength and an polyolens, are highly resistant to solvents and by chlorosulfonic acid and oleum at increase in exibility and elongation-to-break We work in close cooperation with chemicals. room temperature, 98% sulfuric acid, 30% in tension can be expected, depending on our customers to improve existing hydrochloric acid, and 30% hydrogen peroxide the nature and amount of the organic The results of extensive laboratory and actual at 100°C (212°F). They are also affected by 98% medium absorbed. products and develop new resins eld installation tests of polypropylene’s sulfuric acid at 60°C (140°F) and fuming nitric for the rapidly changing markets we chemical resistance are reported in this HMC PP resins have excellent resistance acid and liquid bromine at room temperatures. serve. The company offers a wide downloadable PDF which is periodically to environmental stress-cracking. When Under strain, failure could occur with strong updated. they are tested according to ASTM D1693 range of products suitable for all oxidizing acids at temperatures lower than the brittle fractures that occur with certain major applications and processes, The chemical resistance data presented in this those mentioned. With few exceptions polyethylenes in contact with polar PDF for a range of over 260 chemicals and however, inorganic chemicals produce little or as well as innovative new products organic liquids, detergents, and silicone substances is based on ASTM D543. -

Incompatible Chemical Groups.Pdf
Incompatible Chemical Hazard Groups (and some common examples) Mineral Acids Do NOT Store with… Hydrochloric acid Hydrogen peroxide Acetone Sulfuric Acid Sodium hydroxide Methanol Phosphoric Acid Calcium hydroxide Nitric Acid (keep separate) Chloroform Acetic Acid Strong Organic Acids Do NOT Store with… Acetic Acid3, 4 Hydrogen peroxide Acetone Acetonitrile Formic Acid Sodium hydroxide Methanol Benzene Sulfuric Acid Chloroform Special 1. Organic acids are varied and may be incompatible with each other. Notes: Check MSDSs for specifics 2. Store nitric acid separately in its own secondary container. It is a strong oxidizer. 3. Store acetic acid away from oxidizing agents — especially nitric acid. 4. Acetic acid may be stored with some inorganic acids and most flammable solvents but keep in a separate secondary container. (>70% acetic acid is combustible). Weak These are typically not corrosive and not strongly reactive and can be Organic Acids stored with general liquid lab chemicals. Examples include butyric, maleic, and benzoic acids. Non-Flammable Do NOT Store with… Chlorinated Solvents Methylene chloride Acetone Hexane Chloroform Methanol Nitric Acid Trichloroethane Ethanol Hydrogen Peroxide Carbon tetrachloride Organic Solvents Do NOT Store with… Acetone Hydrogen peroxide Nitric Acid Methanol Sodium hydroxide Chromic Acid Phenol Calcium hydroxide Sulfuric Acid Xylene Trichlorfluoromethane Hydrochloric Acid Oxidizers Do NOT Store with… Nitric Acid Sodium metal Paper and oily rags Hydrogen peroxide Isopropyl Alcohol Xylene Chromic Acid Acetone Sodium nitrate Perchloric Acid Ethyl ether Bromate salts . -

Sd0000029 Evaluation of Chromfte Ore and The
SD0000029 EVALUATION OF CHROMFTE ORE AND THE OPTIMUM METHODS FOR INDUSTRIAL EXTRACTION OF CHROMIUM A TI1HSIS SUBMITTED BY Bakheit Mustafa Mohamed Salih IN CANDIDATURE FOR TIU:DI:GRFJ:OI MASTER OF SC1HNCT DEPARTMENT OF CHEMISTRY FACULTY OF SCIENCE (P.O.BOX 321) OCTOBER 1999 UNIVERSITY OF KHARTOUM 31/ 28 ABSTRACT Samples of chromite ore, collected from Gam and Cheikay mining area (Ingessana Hills) in east Sudan, were analysed to assess the chromium content. Methods for extraction and analysis of chromium metal were developed and established. Analysis were carried out using atomic absorption spectroscopy (AAS) to estimate the contents of chromium, iron, calcium, and magnesium. X-ray fluorescence (XRF) was used to evaluate the levels of chromium, iron, and calcium in the ore. Volumetric analysis was performed to assess chromium and iron, whilst gravimetric analysis was employed to measure the amounts of calcium, magnesium, aluminum and silicon present in the ore. The data was chemically and statistically analyzed to compare the results obtained by the given analytical methods. The results are in good agreement except iron oxide, which displayed a significantly different value when measured by x-ray fluorescence. The data obtained exhibited similarity in almost all cases, when compared with local and global researches, reports, and literature. The study has revealed the average contents of Cr2O3, FeO, CaO, MgO, A12O3, and SiO2 as 40.66. 11.96, 11.94. 0.36. 16.94, 11.45% respectively. MnO and NiO were detected in trace amounts, the corresponding levels in the ore being 72 and 27 ppm. The average chromium content in extracted potassium dichromate measured by using AAS, XRF, and volumetric methods was found to be 31.7%. -

Potassium Dichromate CAS Number: 7778-50-9 EC Number: 231-906-6
21.5.2010 COMMENTS AND RESPONSE TO COMMENTS ON ANNEX XV SVHC: PROPOSAL AND JUSTIFICATION Substance name: Potassium dichromate CAS number: 7778-50-9 EC number: 231-906-6 Reason of the submission of the Annex XV: CMR Disclaimer: The European Chemicals Agency is not responsible for the content of this document. The Response to Comments table has been prepared by the competent authority of the Member State preparing the proposal for identification of a Substance of Very High Concern. The comments were received during the public consultation of the Annex XV dossier. General comments Date Submitted by (name, Comment Response Organisation/MSCA) 20100323 CLEAPSS, UK (on behalf of an CLEAPSS is an advisory service providing support in No answer expected. organisation) science and technology for a consortium of local authorities and their schools including establishments for pupils with special needs. Independent schools, post-16 colleges, teacher training establishments, curriculum developers and others can apply for associate membership. We offer help from nursery education through to A-level studies or equivalent. Our services cover health & safety, risk assessment, sources and use of chemicals, living organisms and equipment. CLEAPSS also provides advice on technicians & their jobs, as well as the design of laboratories and facilities & fittings for D&T and science rooms. 20100324 Germany, company (on behalf of an We reject the ban of Potassium Dichromate in general, Thank for your comment. organisation) because the substance is used in a low concentration (< 1%) in an almost closed system of cuvette tests 20100408 Draeger Safety AG & Co. KGaA, comments k2Cr2O7R1.doc Thank you for this information. -

Reactions of Alcohols
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations – the majority of which are either oxidation or reduction type reactions. Normally: Oxidation is a loss of electrons; Reduction is a gain of electrons. But in organic terms: Oxidation: loss of H2; addition of O or O2; addition of X2 (halogens). Reduction: - addition of H2 or H ; loss of O or O2; loss of X2. Neither an oxidation nor reduction: Addition or loss of H+, H2O, HX. Ch11 Reacns of Alcohols (landscape).docx Page 1 Oxidation of Alcohols Primary and secondary alcohols are easily oxidized by a variety of reagents. Secondary Alcohols The most common reagent used for oxidation of secondary alcohols to ketones is chromic acid, H2CrO4. Chromic acid is produced in situ by reaction of sodium dichromate, sulfuric acid and water. Na2Cr2O7 + H2O + 2H2SO4 2 H2CrO4 + 2 NaHSO4 Ch11 Reacns of Alcohols (landscape).docx Page 2 Mechanism of oxidation The alcohol and chromic acid produce a chromate ester, which then reductively eliminates the Cr species. The Cr is reduced (VI IV), the alcohol is oxidized. Oxidation of Primary Alcohols Primary alcohols are easily oxidized just like secondary alcohols, and the INITIAL product of oxidation is an aldehyde. Ch11 Reacns of Alcohols (landscape).docx Page 3 However, the aldehyde can also be easily oxidized to an acid, and this ‘over-oxidation’ is a practical problem. E.g. A common reagent that selectively oxidizes a primary alcohol to an aldehyde (and no further) is pyridinium chlorochromate, PCC. N: CrO3, HCl (PCC) E.g. Tertiary Alcohols These are resistant to oxidation because they have no hydrogen atoms attached to the oxygen bearing carbon (carbinol carbon).