I26 X-ESR-G-00048 Rev 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emea Public Statement on Thiomersal in Vaccines for Human Use – Recent Evidence Supports Safety of Thiomersal-Containing Vaccines
The European Agency for the Evaluation of Medicinal Products Pre-authorisation evaluation of medicines for human use London, 24 March 2004 Doc. Ref: EMEA/CPMP/VEG/1194/04/Adopted EMEA PUBLIC STATEMENT ON THIOMERSAL IN VACCINES FOR HUMAN USE – RECENT EVIDENCE SUPPORTS SAFETY OF THIOMERSAL-CONTAINING VACCINES The EMEA issued statements on the use of thiomersal in vaccines in 1999 and 2000 (EMEA/20962/99, EMEA/CPMP/1578/00). In light of recent reassuring data on the safety of thiomersal-containing vaccines, this new statement updates previous recommendations. Thiomersal, is an antimicrobial organic mercury compound that continues to be used either in the early stages of manufacturing, or as a preservative, in some vaccines. The antimicrobial action of thiomersal relates to ethylmercury, which is released after breakdown of thiomersal into ethylmercury and thiosalicylate. The Committee for Proprietary Medicinal Products (CPMP) previously advised that although there was no evidence of harm from thiomersal in vaccines other than hypersensitivity (allergic) reactions, it would be prudent to promote the general use of vaccines without thiomersal and other mercury containing preservatives, particularly for single dose vaccines. Since then, several vaccines licensed in the European Union have had thiomersal removed or levels reduced and new vaccines without thiomersal have been licensed. CPMP advice was in line with the global goal of reducing environmental exposure to mercury. The previous assessment of risks associated with ethylmercury had been based on data on methylmercury, as the toxicity profile of the two compounds was assumed to be similar. In March 2004, the CPMP reviewed the latest evidence relating to the safety of thiomersal-containing vaccines. -

PROVISIONAL PEER REVIEWED TOXICITY VALUES for DIMETHYLMERCURY (CASRN 593-74-8) Derivation of Subchronic and Chronic Oral Rfds
EPA/690/R-04/005F l Final 12-01-2004 Provisional Peer Reviewed Toxicity Values for Dimethylmercury (CASRN 593-74-8) Derivation of Subchronic and Chronic Oral RfDs Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 Acronyms and Abbreviations bw body weight cc cubic centimeters CD Caesarean Delivered CERCLA Comprehensive Environmental Response, Compensation and Liability Act of 1980 CNS central nervous system cu.m cubic meter DWEL Drinking Water Equivalent Level FEL frank-effect level FIFRA Federal Insecticide, Fungicide, and Rodenticide Act g grams GI gastrointestinal HEC human equivalent concentration Hgb hemoglobin i.m. intramuscular i.p. intraperitoneal i.v. intravenous IRIS Integrated Risk Information System IUR inhalation unit risk kg kilogram L liter LEL lowest-effect level LOAEL lowest-observed-adverse-effect level LOAEL(ADJ) LOAEL adjusted to continuous exposure duration LOAEL(HEC) LOAEL adjusted for dosimetric differences across species to a human m meter MCL maximum contaminant level MCLG maximum contaminant level goal MF modifying factor mg milligram mg/kg milligrams per kilogram mg/L milligrams per liter MRL minimal risk level i MTD maximum tolerated dose MTL median threshold limit NAAQS National Ambient Air Quality Standards NOAEL no-observed-adverse-effect level NOAEL(ADJ) NOAEL adjusted to continuous exposure duration NOAEL(HEC) NOAEL adjusted for dosimetric differences across species to a -

Mercury in Fish – Background to the Mercury in Fish Advisory Statement
Mercury in fish – Background to the mercury in fish advisory statement (March 2004) Food regulators regularly assess the potential risks associated with the presence of contaminants in the food supply to ensure that, for all sections of the population, these risks are minimised. Food Standards Australia New Zealand (FSANZ) has recently reviewed its risk assessment for mercury in food. The results from this assessment indicate that certain groups, particularly pregnant women, women intending to become pregnant and young children (up to and including 6 years), should limit their consumption of some types of fish in order to control their exposure to mercury. The risk assessment conducted by FSANZ that was published in 2004 used the most recent data and knowledge available at the time. FSANZ intends toreview the advisory statement in the future and will take any new data and scientific evidence into consideration at that time. BENEFITS OF FISH Even though certain types of fish can accumulate higher levels of mercury than others, it is widely recognised that there are considerable nutritional benefits to be derived from the regular consumption of fish. Fish is an excellent source of high biological value protein, is low in saturated fat and contains polyunsaturated fatty acids such as essential omega-3 polyunsaturates. It is also a good source of some vitamins, particularly vitamin D where a 150 g serve of fish will supply around 3 micrograms of vitamin D – about three times the amount of vitamin D in a 10 g serve of margarine. Fish forms a significant component of the Australian diet with approximately 25% of the population consuming fish at least once a week (1995 Australian National Nutrition Survey; McLennan & Podger 1999). -
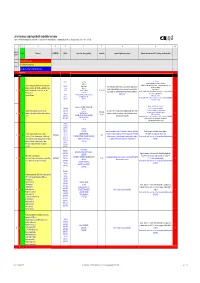
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Inorganic Mercury, Methyl Mercury, Ethyl Mercury
Laboratory Procedure Manual Analyte: Inorganic mercury, Methyl mercury, Ethyl mercury Matrix: Blood Method: Blood mercury speciation TSID-GC-ICP-DRC-MS (Triple Spike Isotope Dilution Gas Chromatography- Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry) Method No: DLS-3020.5 Adopted: Revised: As performed by: Inorganic and Radiation Analytical Toxicology Branch Division of Laboratory Sciences National Center for Environmental Health Contact: Dr. Robert L. Jones Phone: 770-488-7991 Fax: 770-488-4097 Email: [email protected] James L. Pirkle, M.D., Ph.D. Director, Division of Laboratory Sciences Important Information for Users CDC periodically refines these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. Mercury speciation in whole blood NHANES 2011-2012 Page 2 of 52 This document details the Lab Protocol for testing items in the following table: Data File Name Variable Name SAS Label LBXIHG Mercury, inorganic (µg/L) LBDIHGSI Mercury, inorganic (µmol/L) IHgEM_G LBXBGE Mercury, ethyl (µg/dL) LBXBGM Mercury, methyl (μg/L) Mercury speciation in whole blood NHANES 2011-2012 Page 3 of 52 1. CLINICAL RELEVANCE AND TEST PRINCIPLE A. Clinical Relevance Mercury (Hg) is widespread in the environment and found in its elemental form (Hg0), inorganic forms such as mercurous (Hg+) and mercuric (Hg2+), and various organic forms such as methyl mercury (MeHg), ethyl mercury (EtHg), phenyl mercury (PhHg), and others. The health effects of mercury are diverse and depend on the form of mercury encountered and the severity and length of 0 2+ + exposure. -

Methylmercury Contamination: Impacts on Aquatic Systems and Terrestrial Species, and Insights for Abatement
Methylmercury Contamination: Impacts on Aquatic Systems and Terrestrial Species, and Insights for Abatement Charles E. Sams USDA Forest Service, Eastern Region Air Quality Program, Milwaukee, Wisconsin Methylmercury (MeHg) is one of the most widespread waterborne contaminants, and through biomagnification processes has commonly been found in prey fish at concentrations toxic to piscivorous birds and mammals. In USEPA’s National Fish Tissue Survey, the Lowest Adverse Effect Concentration of 0.1 g/g wet weight was exceeded in 28% of total samples and 86% of predatory fish samples (USEPA 2002). Evidence suggests that more than a quarter of all mature fish contain methylmercury concentrations above this level. This review focuses on the leading anthropogenic sources of mercury to aquatic systems, through atmospheric deposition and the environmental dynamics of the mercury methylation process in aquatic sediments. The results of extensive mercury monitoring studies are discussed, as well as the reproductive and behavioral impacts on birds and mammals feeding on fish exhibiting realistic contaminant concentrations. Recommendations are provided for continued research and the abatement of methylmercury concentrations in fish through forest management practices. Keywords: mercury deposition, methylmercury, sulfate reducing bacteria, aquatic sediments INTRODUCTION conterminous United States, over 20.2 million ha (50 million acres) were determined to be forested wetlands, Methylmercury (MeHg) is one of the most widespread as well as 10.0 million ha (25 million acres) of emergent waterborne contaminants (USGS 2001a; UNEP 2002; wetlands and 7.3 million ha (18 million acres) of shrub USDHHS and USEPA 2004). Unhealthful levels of MeHg wetlands (Dahl 2000). While National Forest lands in fish have led to the issuance of fish consumption represent only eight percent of the contiguous United advisories by at least 46 states (USEPA 2004). -

Effects of Dissolved Organic Carbon on Methylmercury Bioavailability in Stream Ecosystems
Effects of dissolved organic carbon on methylmercury bioavailability in stream ecosystems Effects of dissolved organic carbon on methylmercury bioavailability in stream ecosystems Basic Information Effects of dissolved organic carbon on methylmercury bioavailability in stream Title: ecosystems Project Number: 2016NH205G USGS Grant Number: Start Date: 9/1/2016 End Date: 8/31/2018 Funding Source: 104G Congressional 2nd Congressional district of New Hampshire District: Research Category: Water Quality Focus Categories: Surface Water, Geochemical Processes, Toxic Substances Descriptors: None Principal Kathryn L Cottingham, Celia Y. Chen, James Shanley Investigators: Publications There are no publications. 1 Effects of dissolved organic carbon on methylmercury bioavailability in stream ecosystems (2016NH205G) Problem: Neurotoxic methylmercury bioaccumulates through aquatic food webs and is a primary cause for fish consumption advisories in the Northeast. The mobilization, transport and bioavailability of mercury in aquatic ecosystems is strongly tied to organic matter dissolved in the water, yet levels of methylmercury in fish are difficult to predict. Previous studies have noted that relationships between stream methylmercury and dissolved organic carbon (DOC) in streams change over time. There is also a seemingly contradictory effect of DOC on uptake by the biota: at low concentrations of organic carbon, methylmercury bioaccumulation increases, whereas at higher concentrations, uptake into fish is attenuated. This project is testing the hypothesis that differences in the chemical structure of the DOC that is transporting MeHg in streams lead to the temporal changes and non-linearity in bioaccumulation noted in previous studies. Objectives: 1) Identify and characterize fractions of DOC that associate with MeHg and Hg in streams, 2) Determine the effects of DOC quality and quantity on MeHg uptake by primary producers at the base of the stream food web. -

Mercury Cycling in Sulfur Rich Sediment from the Brunswick Estuary
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Summer 2017 Mercury Cycling in Sulfur Rich Sediment From The Brunswick Estuary Travis William Nicolette Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Part of the Environmental Engineering Commons Recommended Citation Nicolette, Travis William, "Mercury Cycling in Sulfur Rich Sediment From The Brunswick Estuary" (2017). Electronic Theses and Dissertations. 1626. https://digitalcommons.georgiasouthern.edu/etd/1626 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. MERCURY CYCLING IN SULFUR RICH SEDIMENTS FROM THE BRUNSWICK ESTUARY by TRAVIS NICOLETTE (Under the direction of major professor Franciscan Cubas) ABSTRACT Mercury is potentially toxic to the environment. Mercury is absorbed into anaerobic sediments of surface waters, which may be converted to methylmercury, a toxic form of mercury that bio-accumulates in aquatic biota. Sources of mercury in the environment vary, but the production of methylmercury is common in sulfur-rich sediments containing mercury. In such environments, sulfur reducing bacteria (SRB) produce methylmercury as a by-product. The metabolic process uses energy from the reduction of sulfate to sulfide. This study focuses on determining the methylmercury production and release potential from sulfur-rich sediments extracted from different areas of the Brunswick Estuary. Previous studies note considerable levels of mercury in the Brunswick Estuary due to a local super fund site. -

Sd0000029 Evaluation of Chromfte Ore and The
SD0000029 EVALUATION OF CHROMFTE ORE AND THE OPTIMUM METHODS FOR INDUSTRIAL EXTRACTION OF CHROMIUM A TI1HSIS SUBMITTED BY Bakheit Mustafa Mohamed Salih IN CANDIDATURE FOR TIU:DI:GRFJ:OI MASTER OF SC1HNCT DEPARTMENT OF CHEMISTRY FACULTY OF SCIENCE (P.O.BOX 321) OCTOBER 1999 UNIVERSITY OF KHARTOUM 31/ 28 ABSTRACT Samples of chromite ore, collected from Gam and Cheikay mining area (Ingessana Hills) in east Sudan, were analysed to assess the chromium content. Methods for extraction and analysis of chromium metal were developed and established. Analysis were carried out using atomic absorption spectroscopy (AAS) to estimate the contents of chromium, iron, calcium, and magnesium. X-ray fluorescence (XRF) was used to evaluate the levels of chromium, iron, and calcium in the ore. Volumetric analysis was performed to assess chromium and iron, whilst gravimetric analysis was employed to measure the amounts of calcium, magnesium, aluminum and silicon present in the ore. The data was chemically and statistically analyzed to compare the results obtained by the given analytical methods. The results are in good agreement except iron oxide, which displayed a significantly different value when measured by x-ray fluorescence. The data obtained exhibited similarity in almost all cases, when compared with local and global researches, reports, and literature. The study has revealed the average contents of Cr2O3, FeO, CaO, MgO, A12O3, and SiO2 as 40.66. 11.96, 11.94. 0.36. 16.94, 11.45% respectively. MnO and NiO were detected in trace amounts, the corresponding levels in the ore being 72 and 27 ppm. The average chromium content in extracted potassium dichromate measured by using AAS, XRF, and volumetric methods was found to be 31.7%. -

Potassium Dichromate CAS Number: 7778-50-9 EC Number: 231-906-6
21.5.2010 COMMENTS AND RESPONSE TO COMMENTS ON ANNEX XV SVHC: PROPOSAL AND JUSTIFICATION Substance name: Potassium dichromate CAS number: 7778-50-9 EC number: 231-906-6 Reason of the submission of the Annex XV: CMR Disclaimer: The European Chemicals Agency is not responsible for the content of this document. The Response to Comments table has been prepared by the competent authority of the Member State preparing the proposal for identification of a Substance of Very High Concern. The comments were received during the public consultation of the Annex XV dossier. General comments Date Submitted by (name, Comment Response Organisation/MSCA) 20100323 CLEAPSS, UK (on behalf of an CLEAPSS is an advisory service providing support in No answer expected. organisation) science and technology for a consortium of local authorities and their schools including establishments for pupils with special needs. Independent schools, post-16 colleges, teacher training establishments, curriculum developers and others can apply for associate membership. We offer help from nursery education through to A-level studies or equivalent. Our services cover health & safety, risk assessment, sources and use of chemicals, living organisms and equipment. CLEAPSS also provides advice on technicians & their jobs, as well as the design of laboratories and facilities & fittings for D&T and science rooms. 20100324 Germany, company (on behalf of an We reject the ban of Potassium Dichromate in general, Thank for your comment. organisation) because the substance is used in a low concentration (< 1%) in an almost closed system of cuvette tests 20100408 Draeger Safety AG & Co. KGaA, comments k2Cr2O7R1.doc Thank you for this information. -

Rpt POL-TOXIC AIR POLLUTANTS 98 BY
SWCAA TOXIC AIR POLLUTANTS '98 by CAS ASIL TAP SQER CAS No HAP POLLUTANT NAME HAP CAT 24hr ug/m3 Ann ug/m3 Class lbs/yr lbs/hr none17 BN 1750 0.20 ALUMINUM compounds none0.00023 AY None None ARSENIC compounds (E649418) ARSENIC COMPOUNDS none0.12 AY 20 None BENZENE, TOLUENE, ETHYLBENZENE, XYLENES BENZENE none0.12 AY 20 None BTEX BENZENE none0.000083 AY None None CHROMIUM (VI) compounds CHROMIUM COMPOUN none0.000083 AY None None CHROMIUM compounds (E649962) CHROMIUM COMPOUN none0.0016 AY 0.5 None COKE OVEN COMPOUNDS (E649830) - CAA 112B COKE OVEN EMISSIONS none3.3 BN 175 0.02 COPPER compounds none0.67 BN 175 0.02 COTTON DUST (raw) none17 BY 1,750 0.20 CYANIDE compounds CYANIDE COMPOUNDS none33 BN 5,250 0.60 FIBROUS GLASS DUST none33 BY 5,250 0.60 FINE MINERAL FIBERS FINE MINERAL FIBERS none8.3 BN 175 0.20 FLUORIDES, as F, containing fluoride, NOS none0.00000003 AY None None FURANS, NITRO- DIOXINS/FURANS none5900 BY 43,748 5.0 HEXANE, other isomers none3.3 BN 175 0.02 IRON SALTS, soluble as Fe none00 AN None None ISOPROPYL OILS none0.5 AY None None LEAD compounds (E650002) LEAD COMPOUNDS none0.4 BY 175 0.02 MANGANESE compounds (E650010) MANGANESE COMPOU none0.33 BY 175 0.02 MERCURY compounds (E650028) MERCURY COMPOUND none33 BY 5,250 0.60 MINERAL FIBERS ((fine), incl glass, glass wool, rock wool, slag w FINE MINERAL FIBERS none0.0021 AY 0.5 None NICKEL 59 (NY059280) NICKEL COMPOUNDS none0.0021 AY 0.5 None NICKEL compounds (E650036) NICKEL COMPOUNDS none0.00000003 AY None None NITROFURANS (nitrofurans furazolidone) DIOXINS/FURANS none0.0013 -

Chromate and the Environment: Removal and Utilization of Industrial Waste
J. Chem. Chem. Eng. 10 (2016) 147-152 doi: 10.17265/1934-7375/2016.03.006 D DAVID PUBLISHING Chromate and the Environment: Removal and Utilization of Industrial Waste Fernando B. Mainier, Pedro Paulo B. Leite, Marcone F. Reis and Thiago Teobaldo Silva Engineering School, Federal Fluminense University(UFF), Niterói, Rio de Janeiro 24220-261, Brazil Abstract: Chromate and dichromate sodium as a function of oxidizer characteristics are used in several industrial areas; for example, in surface protection of coated parts of cadmium, zinc and aluminum (chromate coated treated), corrosion inhibitors, the treatment of leather, the manufacture of pigments, etc. However, the use of such products has been questioned due to the problems of toxicity and pollution that can be caused in the environmental. The Brazilian environmental agency has established that the concentrations of 2- chromate in water courses are less than 0.5 ppm. In order to reuse chromate (CrO4 ) from industrial effluent, laboratory experiments have been proposed based on chemical reduction or electrolytic processes, in order to transform these chromate ions in a final mix of oxides (in solid form), which can then be packed and sent to the production process of sodium chromate. The results of these experiments have become useful industrially (without regard to costs) considering the environmental reuse and the life cycle of the chemical compound. Key words: Chromate, dichromate, contamination, chemical reduction, electrolytic process. 1. Introduction waste, among others [1, 2]. In