Complementary & Alternative Medicine for Mental Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inositol Safety: Clinical Evidences
European Review for Medical and Pharmacological Sciences 2011; 15: 931-936 Inositol safety: clinical evidences G. CARLOMAGNO, V. UNFER AGUNCO Obstetrics & Gynecology Center, Rome (Italy) Abstract. – Myo-inositol is a six carbon ent required by the human cells for the growth cyclitol that contains five equatorial and one axi- and survival in the culture. In humans and other al hydroxyl groups. Myo-inositol has been classi- species, Myo-inositol can be converted to either fied as an insulin sensitizing agent and it is L- or D-chiro-inositol by epimerases. Early stud- commonly used in the treatment of the Polycys- tic Ovary Syndrome (PCOS). However, despite ies showed that inositol urinary clearance was al- its wide clinical use, there is still scarce informa- tered in type 2 diabetes patients, the next step tion on the myo-inositol safety and/or side ef- was to link impaired inositol clearance with in- fects. The aim of the present review was to sum- sulin resistance (for a review see1). Because of marize and discuss available data on the myo-in- these properties, inositol have been classified as ositol safety both in non-clinical and clinical set- “insulin sensitizing agent”2. tings. The main outcome was that only the highest In the recent years, inositol has found more dose of myo-inositol (12 g/day) induced mild and more space in the reproductive clinical prac- gastrointestinal side effects such as nausea, fla- tice3-6. Indeed, since the main therapy for Poly- tus and diarrhea. The severity of side effects did cystic Ovary Syndrome (PCOS) is the use of in- not increase with the dosage. -

Variability of Major Phenyletanes and Phenylpropanoids in 16-Year-Old Rhodiola Rosea L
molecules Article Variability of Major Phenyletanes and Phenylpropanoids in 16-Year-Old Rhodiola rosea L. Clones in Norway Abdelhameed Elameen 1,* , Vera M. Kosman 2, Mette Thomsen 3, Olga N. Pozharitskaya 4 and Alexander N. Shikov 5 1 NIBIO, Norwegian Institute for Bioeconomy Research, Høghskoleveien 7, N-1431 Ås, Norway 2 St. Petersburg Institute of Pharmacy, Leningrad Region, Vsevolozhsky District, P 245 188663 Kuzmolovo, Russia; [email protected] 3 NIBIO, Norwegian Institute for Bioeconomy Research, Øst Apelsvoll, 2849 Kapp, Norway; [email protected] 4 Murmansk Marine Biological Institute of the Russian Academy of Sciences (MMBI RAS), Vladimirskaya, 17, 183010 Murmansk, Russia; [email protected] 5 St. Petersburg State Chemical Pharmaceutical University, Prof. Popov, 14, 197376 Saint-Petersburg, Russia; [email protected] * Correspondence: [email protected]; Tel.: +479-020-0875 Academic Editors: Francesco Cacciola and Lillian Barros Received: 17 June 2020; Accepted: 28 July 2020; Published: 30 July 2020 Abstract: Rhodiola rosea L. (roseroot) is an adaptogen plant belonging to the Crassulaceae family. The broad spectrum of biological activity of R. rosea is attributed to its major phenyletanes and phenylpropanoids: rosavin, salidroside, rosin, cinnamyl alcohol, and tyrosol. In this study, we compared the content of phenyletanes and phenylpropanoids in rhizomes of R. rosea from the Norwegian germplasm collection collected in 2004 and in 2017. In general, the content of these bioactive compounds in 2017 was significantly higher than that observed in 2004. The freeze-drying method increased the concentration of all phenyletanes and phenylpropanoids in rhizomes compared 1 with conventional drying at 70 ◦C. As far as we know, the content of salidroside (51.0 mg g− ) observed in this study is the highest ever detected in Rhodiola spp. -
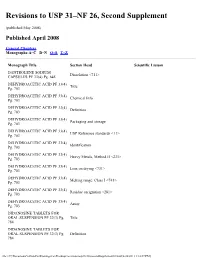
Revisions to USP 31–NF 26, Second Supplement
Revisions to USP 31–NF 26, Second Supplement (published May 2008) Published April 2008 General Chapters Monographs:A–C D–N O–S T–Z Monograph Title Section Head Scientific Liaison DANTROLENE SODIUM Dissolution <711> CAPSULES PF 33(4) Pg. 645 DEHYDROACETIC ACID PF 33(4) Title Pg. 703 DEHYDROACETIC ACID PF 33(4) Chemical Info Pg. 703 DEHYDROACETIC ACID PF 33(4) Definition Pg. 703 DEHYDROACETIC ACID PF 33(4) Packaging and storage Pg. 703 DEHYDROACETIC ACID PF 33(4) USP Reference standards <11> Pg. 703 DEHYDROACETIC ACID PF 33(4) Identification Pg. 703 DEHYDROACETIC ACID PF 33(4) Heavy Metals, Method II <231> Pg. 703 DEHYDROACETIC ACID PF 33(4) Loss on drying <731> Pg. 703 DEHYDROACETIC ACID PF 33(4) Melting range, Class I <741> Pg. 703 DEHYDROACETIC ACID PF 33(4) Residue on ignition <281> Pg. 703 DEHYDROACETIC ACID PF 33(4) Assay Pg. 703 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Title 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Definition 784 file:///C|/Documents%20and%20Settings/rwt/Desktop/revisions/usp31nf26secondSupplement03.html[4/26/2011 1:18:07 PM] DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Packaging and storage 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Labeling 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. USP Reference stadards 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Identification 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Uniformity of dosage units 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Loss on drying 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. -

Vademecum Agrovet Market Animal Health
Vademecum Agrovet Market Animal Health We are a Peruvian company focused on the development, production and commercialization of veterinary products, driving its technical and creative development to meet the needs of veterinarians and stock farmers with unique products of high quality. www.agrovetmarket.com Mission l Products of Unique Class Provide veterinary, nutritional and farmaceutical products of unique class; developed in a creative and innovative way, under high standards of quality that allow us to reach international markets and consolidate locally through the formal stablishment of strategical alliances. Antibiotics Line of large animals am Line of companion animals ac Line of poultry and swine av www.agrovetmarket.com Antibiotics ® Agrogenta 11 | Vetigen 11 Injectable Solution Broad-spectrum aminoglycoside Composition: Gentamicin (as sulphate) 110 mg, excipients q.s. ad 1 mL. Indications: Treatment and prevention of infections caused by microorganisms sensitive to gentamicin (of the urogenital, respiratory and gastrointestinal systems). Also useful in cases of mastitis, metritis, post-operatory and cutaneous infections, septicemias, among others.. Dosage and Administration: Cattle, horse, sheep, goat, swine, camelid: 1 mL/27.5 kg of b.w.; young animals, dogs and cats: 1 mL/14 kg; poultry: 0.1 mL/1.4 kg; every 24 hours for 3 to 5 consecutive days by subcutaneous or intramuscular route. Intrauterine or intra-mammary route: Cows: 2 mL diluted in 20 mL of physiological saline solution for 3 to 5 days. Mares: 20 mL diluted in 200 - 500 mL of physiological saline solution for 3 to 5 days (only intrauterine route). Commercial Presentation: Bottle x 100 mL and 250 mL. -

Qrno. 1 2 3 4 5 6 7 1 CP 2903 77 100 0 Cfcl3
QRNo. General description of Type of Tariff line code(s) affected, based on Detailed Product Description WTO Justification (e.g. National legal basis and entry into Administration, modification of previously the restriction restriction HS(2012) Article XX(g) of the GATT, etc.) force (i.e. Law, regulation or notified measures, and other comments (Symbol in and Grounds for Restriction, administrative decision) Annex 2 of e.g., Other International the Decision) Commitments (e.g. Montreal Protocol, CITES, etc) 12 3 4 5 6 7 1 Prohibition to CP 2903 77 100 0 CFCl3 (CFC-11) Trichlorofluoromethane Article XX(h) GATT Board of Eurasian Economic Import/export of these ozone destroying import/export ozone CP-X Commission substances from/to the customs territory of the destroying substances 2903 77 200 0 CF2Cl2 (CFC-12) Dichlorodifluoromethane Article 46 of the EAEU Treaty DECISION on August 16, 2012 N Eurasian Economic Union is permitted only in (excluding goods in dated 29 may 2014 and paragraphs 134 the following cases: transit) (all EAEU 2903 77 300 0 C2F3Cl3 (CFC-113) 1,1,2- 4 and 37 of the Protocol on non- On legal acts in the field of non- _to be used solely as a raw material for the countries) Trichlorotrifluoroethane tariff regulation measures against tariff regulation (as last amended at 2 production of other chemicals; third countries Annex No. 7 to the June 2016) EAEU of 29 May 2014 Annex 1 to the Decision N 134 dated 16 August 2012 Unit list of goods subject to prohibitions or restrictions on import or export by countries- members of the -

Activity of Selected Group of Monoterpenes in Alzheimer's
International Journal of Molecular Sciences Review Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review Karolina Wojtunik-Kulesza 1,*, Monika Rudkowska 2, Kamila Kasprzak-Drozd 1,* , Anna Oniszczuk 1 and Kinga Borowicz-Reutt 2 1 Department of Inorganic Chemistry, Medical University of Lublin, Chod´zki4a, 20-093 Lublin, Poland; [email protected] 2 Independent Experimental Neuropathophysiology Unit, Medical University of Lublin, Jaczewskiego 8b, 20-090 Lublin, Poland; [email protected] (M.R.); [email protected] (K.B.-R.) * Correspondence: [email protected] (K.W.-K.); [email protected] (K.K.-D.) Abstract: Alzheimer’s disease (AD) is the leading cause of dementia and cognitive function im- pairment. The multi-faced character of AD requires new drug solutions based on substances that incorporate a wide range of activities. Antioxidants, AChE/BChE inhibitors, BACE1, or anti-amyloid platelet aggregation substances are most desirable because they improve cognition with minimal side effects. Plant secondary metabolites, used in traditional medicine and pharmacy, are promising. Among these are the monoterpenes—low-molecular compounds with anti-inflammatory, antioxidant, Citation: Wojtunik-Kulesza, K.; enzyme inhibitory, analgesic, sedative, as well as other biological properties. The presented review Rudkowska, M.; Kasprzak-Drozd, K.; focuses on the pathophysiology of AD and a selected group of anti-neurodegenerative monoterpenes Oniszczuk, A.; Borowicz-Reutt, K. and monoterpenoids for which possible mechanisms of action have been explained. The main body Activity of Selected Group of of the article focuses on monoterpenes that have shown improved memory and learning, anxiolytic Monoterpenes in Alzheimer’s and sleep-regulating effects as determined by in vitro and in silico tests—followed by validation in Disease Symptoms in Experimental in vivo models. -

Hyperforin Inhibits Cell Growth by Inducing Intrinsic and Extrinsic
ANTICANCER RESEARCH 37 : 161-168 (2017) doi:10.21873/anticanres.11301 Hyperforin Inhibits Cell Growth by Inducing Intrinsic and Extrinsic Apoptotic Pathways in Hepatocellular Carcinoma Cells I-TSANG CHIANG 1,2,3* , WEI-TING CHEN 4* , CHIH-WEI TSENG 5, YEN-CHUNG CHEN 2,6 , YU-CHENG KUO 7, BI-JHIH CHEN 8, MAO-CHI WENG 9, HWAI-JENG LIN 10,11# and WEI-SHU WANG 2,12,13# Departments of 1Radiation Oncology, 6Pathology, and 13 Medicine, and 2Cancer Medical Care Center, National Yang-Ming University Hospital, Yilan, Taiwan, R.O.C.; 3Department of Radiological Technology, Central Taiwan University of Science and Technology, Taichung, Taiwan, R.O.C.; 4Department of Psychiatry, Zuoying Branch of Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan, R.O.C.; 5Division of Gastroenterology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chia-Yi, Taiwan, R.O.C.; 7Radiation Oncology, Show Chwan Memorial Hospital, Changhua, Taiwan, R.O.C.; 8Department of Laboratory Medicine, Changhua Christian Hospital, Changhua Christian Medical Foundation, Changhua, Taiwan, R.O.C.; 9Institute of Nuclear Energy Research, Atomic Energy Council, Taoyuan, Taiwan, R.O.C.; 10 Department of Internal Medicine, Division of Gastroenterology and Hepatology, College of Medicine, School of Medicine, Taipei Medical University, Taipei, Taiwan, R.O.C.; 11 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Shuang-Ho Hospital, New Taipei, Taiwan, R.O.C.; 12 National Yang-Ming University School of Medicine, Taipei, Taiwan, R.O.C. Abstract. The aim of the present study was to investigate the (c-FLIP), X-linked inhibitor of apoptosis protein (XIAP), antitumor effect and mechanism of action of hyperforin in myeloid cell leukemia 1(MCL1), and cyclin-D1] were hepatocellular carcinoma (HCC) SK-Hep1 cells in vitro. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

WO 2013/142184 Al 26 September 2013 (26.09.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/142184 Al 26 September 2013 (26.09.2013) P O P C T (51) International Patent Classification: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, A61K 33/16 (2006.01) A61K 31/7048 (2006.01) HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, A61K 33/14 (2006.01) A61K 31/70 (2006.01) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, A61K 33/18 (2006.01) A61K 31/4196 (2006.01) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (21) International Application Number: RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, PCT/US20 13/030788 TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (22) International Filing Date: ZM, ZW. 13 March 2013 (13.03.2013) (84) Designated States (unless otherwise indicated, for every (25) Filing Language: English kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (26) Publication Language: English UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (30) Priority Data: TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 61/612,689 19 March 2012 (19.03.2012) US EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, (71) Applicant: YALE UNIVERSITY [US/US]; Two Whitney TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Avenue, New Haven, CT 065 10 (US). -

Download Supplementary
Supplementary Materials: High throughput virtual screening to discover inhibitors of the main protease of the coronavirus SARS-CoV-2 Olujide O. Olubiyi1,2*, Maryam Olagunju1, Monika Keutmann1, Jennifer Loschwitz1,3, and Birgit Strodel1,3* 1 Institute of Biological Information Processing: Structural Biochemistry, Forschungszentrum Jülich, Jülich, Germany 2 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria 3 Institute of Theoretical and Computational Chemistry, Heinrich Heine University Düsseldorf, 40225 Düsseldorf, Germany * Corresponding authors: [email protected], [email protected] List of Figures S1 Chemical fragments majorly featured in the top performing 9,515 synthetic com- pounds obtained from screening against the crystal structure of the SARS-CoV-2 main protease 3CLpro. .................................. 2 S2 Chemical fragments majorly featured in the top 2,102 synthetic compounds obtained from ensemble docking and application of cutoff values of ∆G ≤ −7.0 kcal/mol and ddyad ≤ 3.5 Å. ............................ 2 S3 The poses and 3CLpro–compound interactions of phthalocyanine and hypericin. 3 S4 The poses and 3CLpro–compound interactions of the four best non-FDA-approved and investigational drugs. ............................... 4 S5 The poses and 3CLpro–compound interactions of zeylanone and glabrolide. 5 List of Tables S1 Names and properties of the compounds binding best to the active site of 3CLpro. 6 1 Supporting Material: High throughput virtual screening for 3CLpro inhibitors Figure S1: Chemical fragments majorly featured in the top performing 9,515 synthetic com- pounds obtained from screening against the crystal structure of the SARS-CoV-2 main pro- tease 3CLpro. The numbers represent the occurrence in absolute numbers. -

APPENDIX a Scoping Letter, NOP, and NOP Comments
APPENDIX A Scoping Letter, NOP, and NOP Comments THE CITY OF SAN DIEGO PLANNING DEPARTMENT Date of Notice: November 24, 2014 PUBLIC NOTICE OF THE PREPARATION OF A PROGRAM ENVIRONMENTAL IMPACT REPORT AND A SCOPING MEETING INTERNAL ORDER No. 21003411 PUBLIC NOTICE: The City of San Diego as the Lead Agency has determined that the project described below will require the preparation of a Program Environmental Impact Report (PEIR) in compliance with the California Environmental Quality Act (CEQA). This Notice of Preparation of a PEIR and Scoping Meeting was publicly noticed and distributed on November 24, 2014. This notice was published in the SAN DIEGO DAILY TRANSCRIPT and placed on the City of San Diego website at: http://www.sandiego.gov/city-clerk/officialdocs/notices/index.shtml SCOPING MEETING: Two public scoping meetings will be held by the City of San Diego's Planning Department one on Tuesday, December 9, 2014 from 5:30 p.m. to 7:30 PM at the South Bay Recreation Center located at 1885 Coronado Avenue, San Diego CA 92154, and one on Thursday, December 11, 2014 from 6:00 PM to 8:00 PM at the Public Utilities Department Metropolitan Operations Complex located at 9192 Topaz Way, San Diego CA 92123. Please note that depending on the number of attendees, the meeting could end earlier than the end times noted above. Verbal and written comments regarding the scope and alternatives of the proposed EIR will be accepted at the meeting. Please send in written/mail-in comments may also be sent to the following address: Myra Herrmann, Environmental Planner, City of San Diego Development Services Center, 1222 First Avenue, MS 501, San Diego, CA 92101 or e-mail your comments to [email protected] with the Project Name and Number in the subject line Number in the subject line within 30 days of the receipt of this notice/date of the Public Notice above. -

St. John's Wort 2018
ONLINE SERIES MONOGRAPHS The Scientific Foundation for Herbal Medicinal Products Hyperici herba St. John's Wort 2018 www.escop.com The Scientific Foundation for Herbal Medicinal Products HYPERICI HERBA St. John's Wort 2018 ESCOP Monographs were first published in loose-leaf form progressively from 1996 to 1999 as Fascicules 1-6, each of 10 monographs © ESCOP 1996, 1997, 1999 Second Edition, completely revised and expanded © ESCOP 2003 Second Edition, Supplement 2009 © ESCOP 2009 ONLINE SERIES ISBN 978-1-901964-61-5 Hyperici herba - St. John's Wort © ESCOP 2018 Published by the European Scientific Cooperative on Phytotherapy (ESCOP) Notaries House, Chapel Street, Exeter EX1 1EZ, United Kingdom www.escop.com All rights reserved Except for the purposes of private study, research, criticism or review no part of this text may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, without the written permission of the publisher. Important Note: Medical knowledge is ever-changing. As new research and clinical experience broaden our knowledge, changes in treatment may be required. In their efforts to provide information on the efficacy and safety of herbal drugs and herbal preparations, presented as a substantial overview together with summaries of relevant data, the authors of the material herein have consulted comprehensive sources believed to be reliable. However, in view of the possibility of human error by the authors or publisher of the work herein, or changes in medical knowledge, neither the authors nor the publisher, nor any other party involved in the preparation of this work, warrants that the information contained herein is in every respect accurate or complete, and they are not responsible for any errors or omissions or for results obtained by the use of such information.