APPENDIX a Scoping Letter, NOP, and NOP Comments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Triptans Step Therapy/Quantity Limit Criteria
Triptans Step Therapy/Quantity Limit Criteria Program may be implemented with the following options 1) step therapy 2) quantity limits or 3) step therapy with quantity limits For Blue Cross and Blue Shield of Illinois Option 1 (step therapy only) will apply. Brand Generic Dosage Form Amerge® naratriptan tablets Axert® almotriptan tablets Frova® frovatriptan tablets Imitrex® sumatriptan injection*, nasal spray, tablets* Maxalt® rizatriptan tablets Maxalt-MLT® rizatriptan tablets Relpax® eletriptan tablets Treximet™ sumatriptan and naproxen tablets Zomig® zolmitriptan tablets, nasal spray Zomig-ZMT® zolmitriptan tablets * generic available and included as target agent in quantity limit edit FDA APPROVED INDICATIONS1-7 The following information is taken from individual drug prescribing information and is provided here as background information only. Not all FDA-approved indications may be considered medically necessary. All criteria are found in the section “Prior Authorization Criteria for Approval.” Amerge® Tablets1, Axert® Tablets2, Frova® Tablets3,Imitrex® injection4, Imitrex Nasal Spray5, Imitrex Tablets6, Maxalt® Tablets7, Maxalt-MLT® Tablets7, Relpax® Tablets8, Treximet™ Tablets9, Zomig® Tablets10, Zomig-ZMT® Tablets10, and Zomig® Nasal Spray11 Amerge (naratriptan), Axert (almotriptan), Frova (frovatriptan), Imitrex (sumatriptan), Maxalt (rizatriptan), Maxalt-MLT (rizatriptan orally disintegrating), Relpax (eletriptan), Treximet (sumatriptan/naproxen), Zomig (zolmitriptan), and Zomig-ZMT (zolmitriptan orally disintegrating) tablets, and Imitrex (sumatriptan) and Zomig (zolmitriptan) nasal spray are all indicated for the acute treatment of migraine attacks with or without aura in adults. They are not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine. Safety and effectiveness of all of these products have not been established for cluster headache, which is present in an older, predominantly male population. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

NEW PATIENT QUESTIONNAIRE - Page 1
__________________________ Clinical Neurosciences Center NEW PATIENT QUESTIONNAIRE - Page 1 Provider you will be seeing: Date of Appointment: Patient Name: Date of Birth: Age: Home Address / City / State / Zip: Home Phone: Work Phone: Cell: Email: Emergency Contact: Phone: PHYSICIAN INFORMATION - What is the name of your PRIMARY CARE PROVIDER: Address / City / State: Phone: What is the name your REFERRING PROVIDER (if different from above): Address / City / State: Phone: HEADACHE SPECIFIC QUESTIONS - What is your biggest concern about your headaches: Do you have sick / severe headaches: YES NO Date sick / severe headache started: How many sick / severe headaches have you had in your life: 0-2 3-10 11-20 21-50 51-100 >100 Frequency of sick / severe headaches (per month and per year): AGE MONTH YEAR DESCRIBE As a child less than 12 years As an adolescent 13-18 years As a young adult 19-30 years As an adult over 30 years Were you adopted: YES NO Does anyone in the family have headaches (migraine, sick, sinus, tension, cluster, other): RELATION YES NO DESCRIBE RELATION YES NO DESCRIBE Mother Father M. Gma P. Gma M. Gpa P. Gpa M. Aunts P. Aunts M. Uncles P. Uncles Sisters Brothers Daughters Sons Were you ever carsick as a child: YES NO NEW PATIENT QUESTIONNAIRE - Page 2 SOME PEOPLE HAVE MORE THAN ONE TYPE OF HEADACHE - How many days have you had a headache in the last: month: days 3 months: days 6 months: days Visits to the ER in the last 12 months: visits Days missed at work or school in the last month: days On a scale of 1-10, on average, -
NARATRIPTAN 2.5 MG FILM-COATED TABLETS [Naratriptan (As Naratriptan Hydrochloride)] Read All of This Leaflet Carefully Before You Start Taking This Medicine
PACKAGE LEAFLET: INFORMATION FOR THE USER NARATRIPTAN 2.5 MG FILM-COATED TABLETS [Naratriptan (as naratriptan hydrochloride)] Read all of this leaflet carefully before you start taking this medicine. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or pharmacist (chemist). • This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their CODE symptoms are the same as yours. CODE • If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. In this leaflet: 1. What Naratriptan is and what it is used for 2. Before you take Naratriptan 3. How to take Naratriptan 4. Possible side effects 5. How to store Naratriptan 6. Further information Zentiva 1. What Naratriptan is and what it is used for Brand: PIL NARATRIPTAN 2.5MG 6 AND 12 FCT 156X348MM Naratriptan contains naratriptan (hydrochloride), which belongs to a group of medicines called triptans (also Category: LEAFLET known as 5-HT1 receptor agonists). Argus Code: 000 Naratriptan tablets are used to treat migraine with or without aura. Spec No: 00000 Migraine symptoms may be caused by the temporary widening of blood vessels in the head. Naratriptan tablets Supersedes: 00000 are believed to reduce the widening of these blood vessels. This in turn helps to take away the headache and Core Spec No: 00000 relieve other symptoms of a migraine attack, such as feeling or being sick (nausea or vomiting) and sensitivity to light and sound. -

Triptan Therapy for Acute Migraine
Triptan Therapy for Acute Migraine Headache John Farr Rothrock, MD University of Alabama at Birmingham, Birmingham, AL Deborah I. Friedman, MD, MPH University of Texas Southwestern, Dallas, TX The “triptans” are 5HT-1B/1D receptor agonists that were developed to treat acute migraine and acute cluster headache. Sumatriptan, the original triptan preparation, has been in general use since 1993, so there has been considerable experience with triptans over time. There are currently seven oral triptans on the market in the United States: sumatriptan (Imitrex™), naratriptan (Amerge™), zolmitriptan (Zomig™), rizatriptan (Maxalt™), almotriptan (Axert™), frovatriptan (Frova™), and eletriptan (Relpax™); brand names may differ by country. There is also a combination preparation of oral sumatriptan/naproxen (Treximet™). Two triptans (sumatriptan, zolmitriptan) are marketed as nasal sprays, and sumatriptan is available for subcutaneous injection, including a needle-free subcutaneous delivery system (Sumavel™). Sumatriptan suppositories are marketed in Europe but not in North America. Zolmitriptan and rizatriptan are sold in an oral disintegrating tablet or “melt” formulation as well as in tablet form; while the “melt” formulations may be more convenient (no liquid is required to propel them into the stomach), they are absorbed similarly to regular tablets and there is no evidence to suggest that they work faster than the tablet formulations. Some patients with migraine-associated nausea prefer the disintegrating tablets while others cannot tolerate their taste. Although all of the triptans initially were investigated for the treatment of migraine headache of moderate to severe intensity and were superior to placebo in those pivotal trials, they appear to be more consistently effective when used to treat migraine earlier in the attack, when the headache is still mild to moderate. -

Triptans: Almotriptan, Amerge®, Axert®, Frova®, Imitrex® and Sumatriptan Tablets/Nasal Spray/Injection, Maxalt/MLT®, Naratriptan, Onzetra™
Drug Therapy Guidelines Triptans: almotriptan, Amerge®, Axert®, Frova®, Imitrex® and sumatriptan tablets/nasal spray/injection, Maxalt/MLT®, naratriptan, Onzetra™ Xsail™, Relpax® and eletriptan tablets, rizatriptan/ODT, Sumavel®, Tosymra™, Treximet®, Zembrace™ SymTouch™, zolmitriptan/ODT tablets, Applicable Zomig/ZMT® tablets/nasal spray Medical Benefit Effective: 5/28/2021 Pharmacy- Formulary 1 x Next Review: 3/22 Pharmacy- Formulary 2 x Date of Origin: 8/29/06 Pharmacy- Formulary 3/Exclusive x Review Dates: 8/06, 11/07, 12/08, 12/09, 6/10, 3/11, 3/12, 3/13, Pharmacy- Formulary 4/AON x 3/14, 3/15, 3/16, 3/17, 3/18, 3/19, 3/20, 2/21, 3/21 I. Medication Description Serotonin receptor agonists are used to treat (not prevent) acute attacks of migraine headaches. These medications work by binding to specific serotonin receptors in the brain which in turn decreases the release of chemicals responsible for the vasodilation of cerebral blood vessels, decreases the activity of pain signaling neurons, and reduces inflammation. II. Position Statement For all lines of business, coverage is provided immediately, without requiring prior authorization, for preferred/formulary medications with associated quantity limit restrictions. Quantity limitations criteria do not apply when the medication is requested by a neurologist. Coverage is determined through a prior authorization process with supporting clinical evidence for all other requests for members aged 18 and older. III. Policy Formulary 1: See Sections B and C Formulary 2: See Sections A and C Formulary 3/Exclusive: See Sections A and C Formulary 4/AON: See Sections A and C A. Non-preferred medications: Amerge, Axert, Frova, Imitrex, Maxalt, Onzetra, Relpax, Tosymra, Treximet, Zembrace, Zomig, Zolmitriptan nasal spray Plan-preferred medications: eletriptan, naratriptan, rizatriptan, sumatriptan, zolmitriptan tablets, zolmitriptan oral disintegrating tablets B. -
![Design, Synthesis and Biological Activity of Novel Dimethyl-{2-[6-Substituted-Indol-1-Yl]-Ethyl}-Amine As Potent, Selective, and Orally-Bioavailable 5-HT1D Agonists](https://docslib.b-cdn.net/cover/5699/design-synthesis-and-biological-activity-of-novel-dimethyl-2-6-substituted-indol-1-yl-ethyl-amine-as-potent-selective-and-orally-bioavailable-5-ht1d-agonists-2655699.webp)
Design, Synthesis and Biological Activity of Novel Dimethyl-{2-[6-Substituted-Indol-1-Yl]-Ethyl}-Amine As Potent, Selective, and Orally-Bioavailable 5-HT1D Agonists
Bioorganic & Medicinal Chemistry Letters 13 (2003) 4409–4413 Design, Synthesis and Biological Activity of Novel Dimethyl-{2-[6-substituted-indol-1-yl]-ethyl}-amine as Potent, Selective, and Orally-Bioavailable 5-HT1D Agonists Methvin Isaac,a,* Malik Slassi,a Tao Xin,a Jalaj Arora,a Anne O’Brien,a Louise Edwards,a Neil MacLean,a Julie Wilson,a Lidia Demschyshyn,a Phillipe Labrie,a Angela Naismith,a Shawn Maddaford,a Damon Papac,b Shuree Harrison,b Hua Wang,b Stan Draperb and Ashok Tehima aNPS Pharmaceuticals Inc., 6850 Goreway Drive, Mississauga, ON, Canada L4V 1V7 bNPS Pharmaceuticals Inc., 420 Chipeta Way, Salt Lake City, UT 84108, USA Received 25 April 2003; accepted 12 September 2003 Abstract—A novel series of highly potent human 5-HT1D agonists, dimethyl-{2-[6-substituted-indol-1-yl]-ethyl}-amine, was syn- thesized. Structure–activity relationship (SAR) investigation revealed 4-[1-(2-dimethylamino-ethyl)-1H-indol-6-yl]-tetrahydro-thio- pyran-4-ol, 11b (ALX-2732), as a potent (Ki=2.4 nM) agonist at the human 5-HT1D receptor with good selectivity over the other serotonin receptor subtypes. This compound demonstrated favorable in vitro metabolic stability in human and rat liver microsomes and was found to be orally bioavailable in rats (Fpo=51%). # 2003 Elsevier Ltd. All rights reserved. The 5-HT1D receptor functions as a terminal autoreceptor trigeminal ganglia, mRNA’s encoding for both h5- regulating the synthesis and release of 5-hydroxy- HT1D and h5-HT1B receptor subtypes (previously tryptamine from serotonergic neurons, and it also acts as termed 5-HT1Da and 5-HT1Db, respectively) appear to a presynaptic heteroreceptor regulating the release of be present. -

Eletriptan in the Management of Acute Migraine
TAN0010.1177/1756285616650619Therapeutic Advances in Neurological DisordersM Capi, M Curto View metadata, citation and similar650619research-article2016 papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza Therapeutic Advances in Neurological Disorders Review Ther Adv Neurol Disord Eletriptan in the management of acute 2016, Vol. 9(5) 414 –423 DOI: 10.1177/ migraine: an update on the evidence for 1756285616650619 © The Author(s), 2016. Reprints and permissions: efficacy, safety, and consistent response http://www.sagepub.co.uk/ journalsPermissions.nav Matilde Capi, Martina Curto, Luana Lionetto, Fernando de Andrés, Giovanna Gentile, Andrea Negro and Paolo Martelletti Abstract: Migraine is a multifactorial, neurological and disabling disorder, also characterized by several autonomic symptoms. Triptans, selective serotonin 5-HT1B/1D agonists, are the first-line treatment option for moderate-to-severe headache attacks. In this paper, we review the recent data on eletriptan clinical efficacy, safety, and tolerability, and potential clinically relevant interactions with other drugs. Among triptans, eletriptan shows a consistent and significant clinical efficacy and a good tolerability profile in the treatment of migraine, Correspondence to: especially for patients with cardiovascular risk factors without coronary artery disease. It Martina Curto, MD Sapienza University shows the most favorable clinical response, together with sumatriptan injections, zolmitriptan of Rome, Azienda and rizatriptan. Additionally, eletriptan shows the most complex pharmacokinetic/dynamic Ospedaliera Sant’Andrea Via di Grottarossa 1035- profile compared with the other triptans. It is metabolized primarily by the CYP3A4 hepatic 1039, Rome 00189, Italy enzyme and therefore the concomitant administration of CYP3A4-potent inhibitors should [email protected] Matilde Capi, MSc be carefully evaluated. -
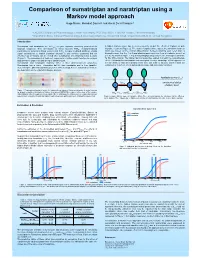
Comparison of Sumatriptan and Naratriptan Using a Markov Model Approach
Comparison of sumatriptan and naratriptan using a Markov model approach Hugo Maasa, Meindert Danhofa and Oscar Della Pasquaa,b aLACDR, Division of Pharmacology, Leiden University, P.O. Box 9502, 2300 RA Leiden, The Netherlands bGlaxoSmithKline, Clinical Pharmacology & Discovery Medicine, Greenford Road, Greenford UB6 0HE, United Kingdom Introduction A hidden Markov model has been developed to predict the effects of triptans on anti- Sumatriptan and naratriptan are 5HT1B/D receptor agonists commonly prescribed for migraine headache. Their mechanism of action involves 5HT1B receptor-mediated migraine treatment (Figure 2). The model comprises three states, the transitions between constriction of intracranial blood vessels and 5HT1D receptor-mediated inhibition of pain which are based on the clinical differentiation between attaining pain relief (from a signal transmission to central trigeminal neurons. It was recently suggested that the headache score 3 or 2 to 1 or 0) and attaining pain resolution (from a headache score 3 or effectiveness of triptans can be limited after sensitisation of central trigeminal neurons 2 to 0). As shown in Figure 1, trigeminal pathophysiology may well be a biological substrate (Figure 1). The assessment of treatment response in migraine ought therefore to consider for this differentiation. The hidden Markov model was applied to predict the concentration- drug access to target sites and timing of administration. effect relationship for sumatriptan and naratriptan. A major advantage of this approach is Sumatriptan and naratriptan distinctly differ in their pharmacokinetic properties. the the ability to estimate transition rates from one state to another, which makes the Sumatriptan has a lower elimination half-life than naratriptan and is less lipophilic. -

Thursday April 29, 1999
4±29±99 Vol. 64 No. 82 Thursday Pages 23007±23164 April 29, 1999 Briefings on how to use the Federal Register For information on briefings in Washington, DC, see announcement on the inside cover of this issue. federal register 1 VerDate 26-APR-99 16:58 Apr 28, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\29APWS.XXX pfrm01 PsN: 29APWS II Federal Register / Vol. 64, No. 82 / Thursday, April 29, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

(Imigran, Suvalan) and Zolmitriptan 2.5 Mg (Zomig) Tablets for Migraine
Naratriptan 2.5 mg (Naramig), sumatriptan 50 mg (Imigran, Suvalan) and zolmitriptan 2.5 mg (Zomig) tablets for migraine Summary PBS listing The maximum quantity has been increased from 2 to 4 tablets. The PBS listing for the triptans (naratriptan, sumatripan and zolmitriptan) is otherwise unchanged. Reason for listing The PBAC recommended an increase in the maximum quantity to cater more adequately for patients who have more than one migraine each month. Place in therapy Triptans should be reserved for patients who have had an adequate trial of a prophylactic agent and in whom other agents have failed to relieve previous attacks. Safety issues There has been no change in the safety considerations for triptans. Serious cardiovascular events have been associated with the triptans; it is therefore particularly important to adhere to the dosing recommendations for this class. Ensure that patients are aware of the appropriate dosing of triptans. Dosing issues There has been no change to the dosing recommendations for triptans. PBS listing The maximum quantity has been increased from 2 to 4 tablets.1 The PBS listing for the triptans (naratriptan, sumatriptan and zolmitriptan) is otherwise unchanged: Authority required Migraine attacks in patients receiving, or who have failed a reasonable trial of, prophylactic medication and where attacks in the past have usually failed to respond to oral therapy with ergotamine and other appropriate agents, or in whom these agents are contraindicated. No applications for increased maximum quantities and/or repeats will be authorised. Reason for listing The PBAC recommended an increase in the maximum quantity to cater more adequately for patients who have more than one migraine each month. -
Medication-Guide.Pdf
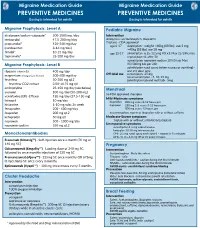
Migraine Medication Guide Migraine Medication Guide PREVENTIVE MEDICINES PREVENTIVE MEDICINES Dosing is intended for adults Dosing is intended for adults Migraine Prophylaxis: Level A Pediatric Migraine divalproex/sodium valproate* 500-1000 mg /day Intervention metoprolol 47.5-200 mg/day Analgesics: acetominophen, ibuprofen propranolol* 120-240 mg/day Triptans - FDA approved age 6-17 rizatriptan - weight <40kg (88 lbs), use 5 mg; (candesartan 8-16 mg/day) >40kg (88 lbs) use 10 mg timolol* 10-15 mg/day age 12-17 almotriptan- 6.25-12.5mg PO x1; Max 25/24h; may topiramate* 25-200 mg/day repeat dose x1 after 2h sumatriptan-naproxen sodium 10/60 tab; Max Migraine Prophylaxis: Level B 85/500mg tab per 24h zolmitriptan nasal spray (when nausea or vomiting) - riboavin (vitamin B2) 400 mg/day one 2.5 dose only Off-label use sumatriptan- 25 mg magnesium trimagnesium dicitrate 500-600 mg/day nasal sumatriptan - 5, 10, 25 mg feverfew 50-300 mg q12 zolmitriptan tab and melt tab - 5mg feverfew CO2 extract 2.08-18.75 mg q8 amitriptyline 25-150 mg/day (see below) Menstrual atenolol 100 mg/day (50-200 mg) no FDA approved therapies venlafaxine (ER) -Effexor 150 mg/day (37.5-150 mg) Mild-Moderate symptoms lisinopril 10 mg/day ibuprofen 800 mg every 8-12 hours prn histamine 1-10 mg subc 2x week naproxen 250 mg 1-2 every 8-12 hours prn fenoprofen 200 - 600 mg/day 500 mg every 12 hours prn acetaminophen, aspirin or ibuprofen with or without caffeine ibuprofen 200 mg q12 ketoprofen 50 mg q8 Moderate-Severe symptoms naproxen 500 - 1000 mg/day triptan with or