H4K20 Monomethylation Faces the WNT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Targeting the Methyltransferase SETD8 Impairs Tumor Cell Survival and Overcomes Drug Resistance Independently of P53 Status in Multiple Myeloma
bioRxiv preprint doi: https://doi.org/10.1101/776930; this version posted September 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Targeting the methyltransferase SETD8 impairs tumor cell survival and overcomes drug resistance independently of p53 status in multiple myeloma Laurie Herviou (1,2,4), Fanny Izard (3,4), Ouissem Karmous-Gadacha (2) , Claire Gourzones (1), Celine Bellanger (1), Eva Desmedt (5), Anqi Ma (6), Laure Vincent (7) , Guillaume Cartron (4,7), Karin Vanderkerken (5), Jian Jin (6), Elke De Bruyne (5), Charlotte Grimaud (3,4,8), Eric Julien (3,4,8 +) and Jérôme Moreaux (1,2,4+) (1) IGH, CNRS, Univ Montpellier, France (2) CHU Montpellier, Laboratory for Monitoring Innovative Therapies, Department of Biological Hematology, Montpellier, France (3) Institut de Recherche en Cancérologie de Montpellier (IRCM), INSERM U1194, Institut Régional du Cancer (ICM), Montpellier F-34298, France (4) University of Montpellier, Montpellier, F-34090, France (5) Department of Hematology and Immunology-Myeloma Center Brussels, Vrije Universiteit Brussel, Brussels, Belgium (6) Mount Sinai Center for Therapeutics Discovery, Departments of Pharmacological Sciences and Oncological Sciences, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, NeW York, NeW York 10029, United States. (7) CHU Montpellier, Department of Clinical Hematology, Montpellier, France (8) Centre National de la Recherche Scientifique (CNRS), F-34293, Montpellier, France + : co-last and corresponding authors ; corresponding authors: Eric Julien ([email protected]) and jérôme Moreaux ([email protected]). 1 bioRxiv preprint doi: https://doi.org/10.1101/776930; this version posted September 20, 2019. -

TRCP Promotes Cell Growth by Targeting PR-Set7/Set8 for Degradation
SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Wang, Z., X. Dai, J. Zhong, H. Inuzuka, L. Wan, X. Li, L. Wang, et al. 2015. “SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation.” Nature Communications 6 (1): 10185. doi:10.1038/ ncomms10185. http://dx.doi.org/10.1038/ncomms10185. Published Version doi:10.1038/ncomms10185 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:23993465 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ARTICLE Received 4 May 2015 | Accepted 12 Nov 2015 | Published 15 Dec 2015 DOI: 10.1038/ncomms10185 OPEN SCFb-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation Zhiwei Wang1,2,*, Xiangpeng Dai2,*, Jiateng Zhong2,3,*, Hiroyuki Inuzuka2, Lixin Wan2, Xiaoning Li2,4, Lixia Wang1, Xiantao Ye1, Liankun Sun4, Daming Gao2,5,LeeZou6 & Wenyi Wei2 The Set8/PR-Set7/KMT5a methyltransferase plays critical roles in governing transcriptional regulation, cell cycle progression and tumorigenesis. Although CRL4Cdt2 was reported to regulate Set8 stability, deleting the PIP motif only led to partial resistance to ultraviolet- induced degradation of Set8, indicating the existence of additional E3 ligase(s) controlling Set8 stability. Furthermore, it remains largely undefined how DNA damage-induced kinase cascades trigger the timely destruction of Set8 to govern tumorigenesis. -

Mechanism of CRL4 , a PCNA-Dependent E3 Ubiquitin Ligase
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase Courtney G. Havens and Johannes C. Walter1 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, USA Eukaryotic cell cycle transitions are driven by E3 ubiq- the ubiquitin is transferred from E1 to the cysteine of an uitin ligases that catalyze the ubiquitylation and destruc- ‘‘E2’’ ubiquitin-conjugating enzyme. Finally, the E2 in- tion of specific protein targets. For example, the anaphase- teracts with an ‘‘E3’’ ubiquitin ligase that also binds the promoting complex/cyclosome (APC/C) promotes the substrate. The juxtaposition of the substrate and the charged exit from mitosis via destruction of securin and mitotic E2 enzyme leads to ubiquitin transfer to the substrate. cyclins, whereas CRL1Skp2 allows entry into S phase by The specificity of ubiquitylation is encoded at the level of targeting the destruction of the cyclin-dependent kinase substrate recognition by the E3 enzymes (Ravid and (CDK) inhibitor p27. Recently, an E3 ubiquitin ligase Hochstrasser 2008); however, recently it has become called CRL4Cdt2 has been characterized, which couples clear that E2s can also contribute to processivity and proteolysis to DNA synthesis via an unusual mechanism specificity for ubiquitin chain nucleation and elongation that involves display of substrate degrons on the DNA (Jin et al. 2008; Ye and Rape 2009; Rodrigo-Brenni et al. polymerase processivity factor PCNA. Through its de- 2010; Saha et al. 2011; Wickliffe et al. 2011). Generally, struction of Cdt1, p21, and Set8, CRL4Cdt2 has emerged as the E3–substrate interaction involves the binding of a master regulator that prevents rereplication in S phase. -

Novel Pharmacological Maps of Protein Lysine Methyltransferases: Key for Target Deorphanization Obdulia Rabal* , Andrea Castellar and Julen Oyarzabal*
Rabal et al. J Cheminform (2018) 10:32 https://doi.org/10.1186/s13321-018-0288-5 RESEARCH ARTICLE Open Access Novel pharmacological maps of protein lysine methyltransferases: key for target deorphanization Obdulia Rabal* , Andrea Castellar and Julen Oyarzabal* Abstract Epigenetic therapies are being investigated for the treatment of cancer, cognitive disorders, metabolic alterations and autoinmune diseases. Among the diferent epigenetic target families, protein lysine methyltransferases (PKMTs), are especially interesting because it is believed that their inhibition may be highly specifc at the functional level. Despite its relevance, there are currently known inhibitors against only 10 out of the 50 SET-domain containing members of the PKMT family. Accordingly, the identifcation of chemical probes for the validation of the therapeutic impact of epigenetic modulation is key. Moreover, little is known about the mechanisms that dictate their substrate specifc- ity and ligand selectivity. Consequently, it is desirable to explore novel methods to characterize the pharmacologi- cal similarity of PKMTs, going beyond classical phylogenetic relationships. Such characterization would enable the prediction of ligand of-target efects caused by lack of ligand selectivity and the repurposing of known compounds against alternative targets. This is particularly relevant in the case of orphan targets with unreported inhibitors. Here, we frst perform a systematic study of binding modes of cofactor and substrate bound ligands with all available SET domain-containing PKMTs. Protein ligand interaction fngerprints were applied to identify conserved hot spots and contact-specifc residues across subfamilies at each binding site; a relevant analysis for guiding the design of novel, selective compounds. Then, a recently described methodology (GPCR-CoINPocket) that incorporates ligand contact information into classical alignment-based comparisons was applied to the entire family of 50 SET-containing proteins to devise pharmacological similarities between them. -

Understanding the Histone DNA Repair Code: H4k20me2 Makes Its Mark
Author Manuscript Published OnlineFirst on June 1, 2018; DOI: 10.1158/1541-7786.MCR-17-0688 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Regulation and Function of H4K20me2 in DNA Repair Understanding the histone DNA repair code: H4K20me2 makes its mark Karissa L. Paquina and Niall G. Howletta,1 aDepartment of Cell and Molecular Biology, University of Rhode Island, Kingston, Rhode Island, U.S.A 1Corresponding author: Niall G. Howlett Ph.D., 379 Center for Biotechnology and Life Sciences, 120 Flagg Road, Kingston, RI, USA, Tel.: +1 401 874 4306; Fax: +1 401 874 2065; Email address: [email protected] Keywords: H4K20me2, DNA DSB repair, histone posttranslational modification, homologous recombination, nonhomologous DNA end joining The authors declare no potential conflicts of interest 1 Downloaded from mcr.aacrjournals.org on September 26, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 1, 2018; DOI: 10.1158/1541-7786.MCR-17-0688 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Regulation and Function of H4K20me2 in DNA Repair Abstract Chromatin is a highly compact structure that must be rapidly rearranged in order for DNA repair proteins to access sites of damage and facilitate timely and efficient repair. Chromatin plasticity is achieved through multiple processes, including the post- translational modification of histone tails. In recent years, the impact of histone post- translational modification on the DNA damage response has become increasingly well recognized, and chromatin plasticity has been firmly linked to efficient DNA repair. -

Histone 4 Lysine 20 Methylation: a Case for Neurodevelopmental Disease
biology Review Histone 4 Lysine 20 Methylation: A Case for Neurodevelopmental Disease Rochelle N. Wickramasekara and Holly A. F. Stessman * Department of Pharmacology, School of Medicine, Creighton University, Omaha, NE 68178, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-402-280-2255 Received: 28 December 2018; Accepted: 26 February 2019; Published: 3 March 2019 Abstract: Neurogenesis is an elegantly coordinated developmental process that must maintain a careful balance of proliferation and differentiation programs to be compatible with life. Due to the fine-tuning required for these processes, epigenetic mechanisms (e.g., DNA methylation and histone modifications) are employed, in addition to changes in mRNA transcription, to regulate gene expression. The purpose of this review is to highlight what we currently know about histone 4 lysine 20 (H4K20) methylation and its role in the developing brain. Utilizing publicly-available RNA-Sequencing data and published literature, we highlight the versatility of H4K20 methyl modifications in mediating diverse cellular events from gene silencing/chromatin compaction to DNA double-stranded break repair. From large-scale human DNA sequencing studies, we further propose that the lysine methyltransferase gene, KMT5B (OMIM: 610881), may fit into a category of epigenetic modifier genes that are critical for typical neurodevelopment, such as EHMT1 and ARID1B, which are associated with Kleefstra syndrome (OMIM: 610253) and Coffin-Siris syndrome (OMIM: 135900), respectively. Based on our current knowledge of the H4K20 methyl modification, we discuss emerging themes and interesting questions on how this histone modification, and particularly KMT5B expression, might impact neurodevelopment along with current challenges and potential avenues for future research. -

The Emerging Role for Cullin 4 Family of E3 Ligases in Tumorigenesis T ⁎ ⁎ Ji Chenga,B,1, Jianping Guob,1, Brian J
BBA - Reviews on Cancer 1871 (2019) 138–159 Contents lists available at ScienceDirect BBA - Reviews on Cancer journal homepage: www.elsevier.com/locate/bbacan Review The emerging role for Cullin 4 family of E3 ligases in tumorigenesis T ⁎ ⁎ Ji Chenga,b,1, Jianping Guob,1, Brian J. Northb, Kaixiong Taoa, Pengbo Zhouc, , Wenyi Weib, a Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China b Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA c Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, 1300 York Ave., New York, NY 10065, USA ARTICLE INFO ABSTRACT Keywords: As a member of the Cullin-RING ligase family, Cullin-RING ligase 4 (CRL4) has drawn much attention due to its CRL4, Cullin 4 broad regulatory roles under physiological and pathological conditions, especially in neoplastic events. Based on E3 ligases evidence from knockout and transgenic mouse models, human clinical data, and biochemical interactions, we PROTACs summarize the distinct roles of the CRL4 E3 ligase complexes in tumorigenesis, which appears to be tissue- and Tumorigenesis context-dependent. Notably, targeting CRL4 has recently emerged as a noval anti-cancer strategy, including Targeted therapy thalidomide and its derivatives that bind to the substrate recognition receptor cereblon (CRBN), and anticancer sulfonamides that target DCAF15 to suppress the neoplastic proliferation of multiple myeloma and colorectal cancers, respectively. To this end, PROTACs have been developed as a group of engineered bi-functional che- mical glues that induce the ubiquitination-mediated degradation of substrates via recruiting E3 ligases, such as CRL4 (CRBN) and CRL2 (pVHL). -

A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2018 A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family Linda Molla Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy by Linda Molla June 2018 © Copyright by Linda Molla 2018 A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY Linda Molla, Ph.D. The Rockefeller University 2018 APOBEC2 is a member of the AID/APOBEC cytidine deaminase family of proteins. Unlike most of AID/APOBEC, however, APOBEC2’s function remains elusive. Previous research has implicated APOBEC2 in diverse organisms and cellular processes such as muscle biology (in Mus musculus), regeneration (in Danio rerio), and development (in Xenopus laevis). APOBEC2 has also been implicated in cancer. However the enzymatic activity, substrate or physiological target(s) of APOBEC2 are unknown. For this thesis, I have combined Next Generation Sequencing (NGS) techniques with state-of-the-art molecular biology to determine the physiological targets of APOBEC2. Using a cell culture muscle differentiation system, and RNA sequencing (RNA-Seq) by polyA capture, I demonstrated that unlike the AID/APOBEC family member APOBEC1, APOBEC2 is not an RNA editor. Using the same system combined with enhanced Reduced Representation Bisulfite Sequencing (eRRBS) analyses I showed that, unlike the AID/APOBEC family member AID, APOBEC2 does not act as a 5-methyl-C deaminase. -
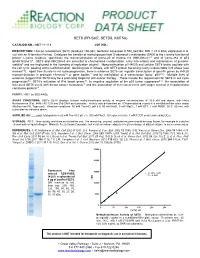
SET8 (PR-Set7, SETD8, KMT5A)
SET8 (PR-Set7, SETD8, KMT5A) CATALOG NO.: HMT-11-118 LOT NO.: DESCRIPTION: Human recombinant SET8 (residues 190-352; Genbank Accession # NM_020382; MW = 21.8 kDa) expressed in E. coli with an N-terminal His-tag. Catalyzes the transfer of methyl groups from S-adenosyl-L-methionine (SAM) to the ε-amino function of protein L-lysine residues, specifically the monomethylation of lysine-20 of histone H4 (H4K20me1)1-3 and of lysine-382 of p53 (p53K382me1)4. SET8 and H4K20me1 are essential to chromosome condensation, entry into mitosis and maintenance of genomic stability5 and are implicated in the licensing of replication origins6. Monomethylation of H4K20 and cellular SET8 levels oscillate with the cell cycle, peaking at the G2/M transition, declining late in mitosis, with SET8 protein becoming nearly undetectable in S phase (see reviews7-9). Apart from its role in cell cycle progression, there is evidence SET8 can regulate transcription of specific genes by H4K20 monomethylation in promoter elements10 or gene bodies11 and by methylation of a transcription factor, p534,12. Multiple lines of evidence suggest that SET8 may be a promising target for anti-cancer therapy. These include the requirement for SET8 in cell cycle progression5,6, SET8’s activation of Wnt target genes10, its negative regulation of the p53 tumor suppressor4,12, the association of increased SET8 levels with breast cancer metastasis13 and the association of decreased levels with longer survival in hepatocellular carcinoma patients14. PURITY: >90% by SDS-PAGE. ASSAY CONDITIONS: RBC’s SET8 displays histone methyltransferase activity at enzyme concentrations of 15.6 nM and above, with HeLa Nucleosomes (Cat. -

Role of Histone Methylation in Maintenance of Genome Integrity
G C A T T A C G G C A T genes Review Role of Histone Methylation in Maintenance of Genome Integrity Arjamand Mushtaq 1, Ulfat Syed Mir 1, Clayton R. Hunt 2, Shruti Pandita 3, Wajahat W. Tantray 1, Audesh Bhat 4, Raj K. Pandita 2, Mohammad Altaf 1,5,* and Tej K. Pandita 2,6,* 1 Chromatin and Epigenetics Lab, Department of Biotechnology, University of Kashmir, Srinagar 190006, Jammu and Kashmir, India; [email protected] (A.M.); [email protected] (U.S.M.); [email protected] (W.W.T.) 2 Houston Methodist Research Institute, Houston, TX 77030, USA; [email protected] (C.R.H.); [email protected] (R.K.P.) 3 Division of Hematology and Medical Oncology, Saint Louis University, St. Louis, MO 63110, USA; [email protected] 4 Centre for Molecular Biology, Central University of Jammu, Bagla 181143, Jammu and Kashmir, India; [email protected] 5 Centre for Interdisciplinary Research and Innovations, University of Kashmir, Srinagar 190006, Jammu and Kashmir, India 6 Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030, USA * Correspondence: [email protected] (M.A.); [email protected] (T.K.P.) Abstract: Packaging of the eukaryotic genome with histone and other proteins forms a chromatin structure that regulates the outcome of all DNA mediated processes. The cellular pathways that ensure genomic stability detect and repair DNA damage through mechanisms that are critically dependent upon chromatin structures established by histones and, particularly upon transient histone Citation: Mushtaq, A.; Mir, U.S.; post-translational modifications. Though subjected to a range of modifications, histone methylation Hunt, C.R.; Pandita, S.; Tantray, W.W.; is especially crucial for DNA damage repair, as the methylated histones often form platforms for Bhat, A.; Pandita, R.K.; Altaf, M.; Pandita, T.K. -

Structural Origins for the Product Specificity of SET Domain Protein Methyltransferases
Structural origins for the product specificity of SET domain protein methyltransferases Jean-Franc¸ois Couturea,1, Lynnette M. A. Dirkb, Joseph S. Brunzellec, Robert L. Houtzb, and Raymond C. Trievela,2 aDepartment of Biological Chemistry, University of Michigan, Ann Arbor, MI 48109; bDepartment of Horticulture, Plant Physiology/Biochemistry/Molecular Biology Program, University of Kentucky, Lexington, KY 40546; and cDepartment of Molecular Pharmacology and Biological Chemistry, Life Sciences Collaborative Access Team, Northwestern University Center for Synchrotron Research, Argonne, IL 60439 Edited by David R. Davies, National Institutes of Health, Bethesda, MD, and approved October 30, 2008 (received for review July 11, 2008) SET domain protein lysine methyltransferases (PKMTs) regulate histone methyltransferases (Fig. S1). In lysine monomethyltrans- transcription and other cellular functions through site-specific ferases, this position is occupied by a tyrosine, whereas in most methylation of histones and other substrates. PKMTs catalyze the dimethyltransferases and trimethyltransferases, a phenylalanine formation of monomethylated, dimethylated, or trimethylated or another hydrophobic amino acid is located in this site (5–10). products, establishing an additional hierarchy with respect to These findings underpin a Phe/Tyr switch model for product methyllysine recognition in signaling. Biochemical studies of PK- specificity wherein the identity of the residue occupying the MTs have identified a conserved position within their active sites, switch position differentiates monomethyltransferases and di/ the Phe/Tyr switch, that governs their respective product specific- trimethyltransferases, with the exception of enzymes that are ities. To elucidate the mechanism underlying this switch, we have active within the context of heteromeric complexes whose sub- characterized a Phe/Tyr switch mutant of the histone H4 Lys-20 units regulate product specificity, such as ScSET1 (11) and (H4K20) methyltransferase SET8, which alters its specificity from a HsMLL (12, 13).