Histone 4 Lysine 20 Methylation: a Case for Neurodevelopmental Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Targeting the Methyltransferase SETD8 Impairs Tumor Cell Survival and Overcomes Drug Resistance Independently of P53 Status in Multiple Myeloma
bioRxiv preprint doi: https://doi.org/10.1101/776930; this version posted September 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Targeting the methyltransferase SETD8 impairs tumor cell survival and overcomes drug resistance independently of p53 status in multiple myeloma Laurie Herviou (1,2,4), Fanny Izard (3,4), Ouissem Karmous-Gadacha (2) , Claire Gourzones (1), Celine Bellanger (1), Eva Desmedt (5), Anqi Ma (6), Laure Vincent (7) , Guillaume Cartron (4,7), Karin Vanderkerken (5), Jian Jin (6), Elke De Bruyne (5), Charlotte Grimaud (3,4,8), Eric Julien (3,4,8 +) and Jérôme Moreaux (1,2,4+) (1) IGH, CNRS, Univ Montpellier, France (2) CHU Montpellier, Laboratory for Monitoring Innovative Therapies, Department of Biological Hematology, Montpellier, France (3) Institut de Recherche en Cancérologie de Montpellier (IRCM), INSERM U1194, Institut Régional du Cancer (ICM), Montpellier F-34298, France (4) University of Montpellier, Montpellier, F-34090, France (5) Department of Hematology and Immunology-Myeloma Center Brussels, Vrije Universiteit Brussel, Brussels, Belgium (6) Mount Sinai Center for Therapeutics Discovery, Departments of Pharmacological Sciences and Oncological Sciences, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, NeW York, NeW York 10029, United States. (7) CHU Montpellier, Department of Clinical Hematology, Montpellier, France (8) Centre National de la Recherche Scientifique (CNRS), F-34293, Montpellier, France + : co-last and corresponding authors ; corresponding authors: Eric Julien ([email protected]) and jérôme Moreaux ([email protected]). 1 bioRxiv preprint doi: https://doi.org/10.1101/776930; this version posted September 20, 2019. -

TRCP Promotes Cell Growth by Targeting PR-Set7/Set8 for Degradation
SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Wang, Z., X. Dai, J. Zhong, H. Inuzuka, L. Wan, X. Li, L. Wang, et al. 2015. “SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation.” Nature Communications 6 (1): 10185. doi:10.1038/ ncomms10185. http://dx.doi.org/10.1038/ncomms10185. Published Version doi:10.1038/ncomms10185 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:23993465 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ARTICLE Received 4 May 2015 | Accepted 12 Nov 2015 | Published 15 Dec 2015 DOI: 10.1038/ncomms10185 OPEN SCFb-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation Zhiwei Wang1,2,*, Xiangpeng Dai2,*, Jiateng Zhong2,3,*, Hiroyuki Inuzuka2, Lixin Wan2, Xiaoning Li2,4, Lixia Wang1, Xiantao Ye1, Liankun Sun4, Daming Gao2,5,LeeZou6 & Wenyi Wei2 The Set8/PR-Set7/KMT5a methyltransferase plays critical roles in governing transcriptional regulation, cell cycle progression and tumorigenesis. Although CRL4Cdt2 was reported to regulate Set8 stability, deleting the PIP motif only led to partial resistance to ultraviolet- induced degradation of Set8, indicating the existence of additional E3 ligase(s) controlling Set8 stability. Furthermore, it remains largely undefined how DNA damage-induced kinase cascades trigger the timely destruction of Set8 to govern tumorigenesis. -
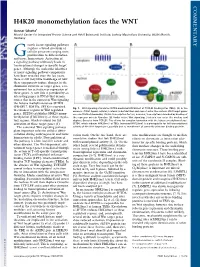
H4K20 Monomethylation Faces the WNT
COMMENTARY H4K20 monomethylation faces the WNT Gunnar Schotta1 Munich Center for Integrated Protein Science and Adolf Butenandt Institute, Ludwig Maximilians University, 80336 Munich, Germany rowth factor signaling pathways regulate a broad spectrum of G cellular processes ranging from proliferation to differentiation and tissue homeostasis. Activation of a signaling pathway ultimately leads to transcriptional changes in specific target genes. Although the molecular identities of many signaling pathway components have been revealed over the last years, there is still very little knowledge of how these components induce changes in the chromatin structure of target genes, a re- quirement for activation or repression of these genes. A new link is provided by an interesting paper in PNAS that demon- strates that in the context of Wnt signaling the histone methyltransferase SETD8 (PR-SET7, KMT5a, SET8) is recruited Fig. 1. Wnt signaling stimulates SETD8-mediated H4K20me1 at TCF/LEF binding sites (TBEs). (A)Inthe to enhancer regions of Wnt-regulated absence of Wnt ligand, cellular β-catenin is destabilized and cannot enter the nucleus. Wnt target genes genes. SETD8 establishes H4K20 mono- are constitutively bound by TCF/LEF transcription factors; however, transcription is blocked by binding of methylation (H4K20me1) at these regula- the repressor protein Groucho. (B) Under active Wnt signaling, β-catenin can enter the nucleus and tory regions, which is crucial for full displace Groucho from TCF/LEF. This allows for complex formation with the histone methyltransferase activation of these target genes (1). SETD8, which induces H4K20me1 at TBEs. Increased H4K20me1 is a prerequisite for full transcriptional The canonical Wnt signaling pathway activity of the Wnt target gene, possibly due to recruitment of currently unknown binding proteins. -

Sleeping Beauty Transposon Mutagenesis Identifies Genes That
Sleeping Beauty transposon mutagenesis identifies PNAS PLUS genes that cooperate with mutant Smad4 in gastric cancer development Haruna Takedaa,b, Alistair G. Rustc,d, Jerrold M. Warda, Christopher Chin Kuan Yewa, Nancy A. Jenkinsa,e, and Neal G. Copelanda,e,1 aDivision of Genomics and Genetics, Institute of Molecular and Cell Biology, Agency for Science, Technology and Research, Singapore 138673; bDepartment of Pathology, School of Medicine, Kanazawa Medical University, Ishikawa 920-0293, Japan; cExperimental Cancer Genetics, Wellcome Trust Sanger Institute, Cambridge CB10 1HH, United Kingdom; dTumour Profiling Unit, The Institute of Cancer Research, Chester Beatty Laboratories, London SW3 6JB, United Kingdom; and eCancer Research Program, Houston Methodist Research Institute, Houston, TX 77030 Contributed by Neal G. Copeland, February 27, 2016 (sent for review October 15, 2015; reviewed by Yoshiaki Ito and David A. Largaespada) Mutations in SMAD4 predispose to the development of gastroin- animal models that mimic human GC, researchers have infected testinal cancer, which is the third leading cause of cancer-related mice with H. pylori and then, treated them with carcinogens. They deaths. To identify genes driving gastric cancer (GC) development, have also used genetic engineering to develop a variety of trans- we performed a Sleeping Beauty (SB) transposon mutagenesis genic and KO mouse models of GC (10). Smad4 KO mice are one + − screen in the stomach of Smad4 / mutant mice. This screen iden- GC model that has been of particular interest to us (11, 12). tified 59 candidate GC trunk drivers and a much larger number of Heterozygous Smad4 KO mice develop polyps in the pyloric re- candidate GC progression genes. -

Human Brain Organoids Reveal Accelerated Development of Cortical Neuron Classes As a Shared Feature of Autism Risk Genes
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.10.376509; this version posted November 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Human brain organoids reveal accelerated development of cortical neuron classes as a shared feature of autism risk genes Bruna Paulsen1,2,†, Silvia Velasco1,2,†,#, Amanda J. Kedaigle1,2,3,†, Martina Pigoni1,2,†, Giorgia Quadrato4,5 Anthony Deo2,6,7,8, Xian Adiconis2,3, Ana Uzquiano1,2, Kwanho Kim1,2,3, Sean K. Simmons2,3, Kalliopi Tsafou2, Alex Albanese9, Rafaela Sartore1,2, Catherine Abbate1,2, Ashley Tucewicz1,2, Samantha Smith1,2, Kwanghun Chung9,10,11,12, Kasper Lage2,13, Aviv Regev3,14, Joshua Z. Levin2,3, Paola Arlotta1,2,# † These authors contributed equally to the work # Correspondence should be addressed to [email protected] and [email protected] 1 Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, MA 02138, USA 2 Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA 3 Klarman Cell Observatory, Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA 4 Department of Stem Cell Biology and Regenerative Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA; 5 Eli and Edythe Broad CIRM Center for Regenerative Medicine and Stem Cell Research at the University of Southern California, Los Angeles, CA 90033, USA. 6 Department of Psychiatry, -

Mechanism of CRL4 , a PCNA-Dependent E3 Ubiquitin Ligase
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase Courtney G. Havens and Johannes C. Walter1 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, USA Eukaryotic cell cycle transitions are driven by E3 ubiq- the ubiquitin is transferred from E1 to the cysteine of an uitin ligases that catalyze the ubiquitylation and destruc- ‘‘E2’’ ubiquitin-conjugating enzyme. Finally, the E2 in- tion of specific protein targets. For example, the anaphase- teracts with an ‘‘E3’’ ubiquitin ligase that also binds the promoting complex/cyclosome (APC/C) promotes the substrate. The juxtaposition of the substrate and the charged exit from mitosis via destruction of securin and mitotic E2 enzyme leads to ubiquitin transfer to the substrate. cyclins, whereas CRL1Skp2 allows entry into S phase by The specificity of ubiquitylation is encoded at the level of targeting the destruction of the cyclin-dependent kinase substrate recognition by the E3 enzymes (Ravid and (CDK) inhibitor p27. Recently, an E3 ubiquitin ligase Hochstrasser 2008); however, recently it has become called CRL4Cdt2 has been characterized, which couples clear that E2s can also contribute to processivity and proteolysis to DNA synthesis via an unusual mechanism specificity for ubiquitin chain nucleation and elongation that involves display of substrate degrons on the DNA (Jin et al. 2008; Ye and Rape 2009; Rodrigo-Brenni et al. polymerase processivity factor PCNA. Through its de- 2010; Saha et al. 2011; Wickliffe et al. 2011). Generally, struction of Cdt1, p21, and Set8, CRL4Cdt2 has emerged as the E3–substrate interaction involves the binding of a master regulator that prevents rereplication in S phase. -

Novel Pharmacological Maps of Protein Lysine Methyltransferases: Key for Target Deorphanization Obdulia Rabal* , Andrea Castellar and Julen Oyarzabal*
Rabal et al. J Cheminform (2018) 10:32 https://doi.org/10.1186/s13321-018-0288-5 RESEARCH ARTICLE Open Access Novel pharmacological maps of protein lysine methyltransferases: key for target deorphanization Obdulia Rabal* , Andrea Castellar and Julen Oyarzabal* Abstract Epigenetic therapies are being investigated for the treatment of cancer, cognitive disorders, metabolic alterations and autoinmune diseases. Among the diferent epigenetic target families, protein lysine methyltransferases (PKMTs), are especially interesting because it is believed that their inhibition may be highly specifc at the functional level. Despite its relevance, there are currently known inhibitors against only 10 out of the 50 SET-domain containing members of the PKMT family. Accordingly, the identifcation of chemical probes for the validation of the therapeutic impact of epigenetic modulation is key. Moreover, little is known about the mechanisms that dictate their substrate specifc- ity and ligand selectivity. Consequently, it is desirable to explore novel methods to characterize the pharmacologi- cal similarity of PKMTs, going beyond classical phylogenetic relationships. Such characterization would enable the prediction of ligand of-target efects caused by lack of ligand selectivity and the repurposing of known compounds against alternative targets. This is particularly relevant in the case of orphan targets with unreported inhibitors. Here, we frst perform a systematic study of binding modes of cofactor and substrate bound ligands with all available SET domain-containing PKMTs. Protein ligand interaction fngerprints were applied to identify conserved hot spots and contact-specifc residues across subfamilies at each binding site; a relevant analysis for guiding the design of novel, selective compounds. Then, a recently described methodology (GPCR-CoINPocket) that incorporates ligand contact information into classical alignment-based comparisons was applied to the entire family of 50 SET-containing proteins to devise pharmacological similarities between them. -

Comparative Transcriptomics Reveals Similarities and Differences
Seifert et al. BMC Cancer (2015) 15:952 DOI 10.1186/s12885-015-1939-9 RESEARCH ARTICLE Open Access Comparative transcriptomics reveals similarities and differences between astrocytoma grades Michael Seifert1,2,5*, Martin Garbe1, Betty Friedrich1,3, Michel Mittelbronn4 and Barbara Klink5,6,7 Abstract Background: Astrocytomas are the most common primary brain tumors distinguished into four histological grades. Molecular analyses of individual astrocytoma grades have revealed detailed insights into genetic, transcriptomic and epigenetic alterations. This provides an excellent basis to identify similarities and differences between astrocytoma grades. Methods: We utilized public omics data of all four astrocytoma grades focusing on pilocytic astrocytomas (PA I), diffuse astrocytomas (AS II), anaplastic astrocytomas (AS III) and glioblastomas (GBM IV) to identify similarities and differences using well-established bioinformatics and systems biology approaches. We further validated the expression and localization of Ang2 involved in angiogenesis using immunohistochemistry. Results: Our analyses show similarities and differences between astrocytoma grades at the level of individual genes, signaling pathways and regulatory networks. We identified many differentially expressed genes that were either exclusively observed in a specific astrocytoma grade or commonly affected in specific subsets of astrocytoma grades in comparison to normal brain. Further, the number of differentially expressed genes generally increased with the astrocytoma grade with one major exception. The cytokine receptor pathway showed nearly the same number of differentially expressed genes in PA I and GBM IV and was further characterized by a significant overlap of commonly altered genes and an exclusive enrichment of overexpressed cancer genes in GBM IV. Additional analyses revealed a strong exclusive overexpression of CX3CL1 (fractalkine) and its receptor CX3CR1 in PA I possibly contributing to the absence of invasive growth. -

Understanding the Histone DNA Repair Code: H4k20me2 Makes Its Mark
Author Manuscript Published OnlineFirst on June 1, 2018; DOI: 10.1158/1541-7786.MCR-17-0688 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Regulation and Function of H4K20me2 in DNA Repair Understanding the histone DNA repair code: H4K20me2 makes its mark Karissa L. Paquina and Niall G. Howletta,1 aDepartment of Cell and Molecular Biology, University of Rhode Island, Kingston, Rhode Island, U.S.A 1Corresponding author: Niall G. Howlett Ph.D., 379 Center for Biotechnology and Life Sciences, 120 Flagg Road, Kingston, RI, USA, Tel.: +1 401 874 4306; Fax: +1 401 874 2065; Email address: [email protected] Keywords: H4K20me2, DNA DSB repair, histone posttranslational modification, homologous recombination, nonhomologous DNA end joining The authors declare no potential conflicts of interest 1 Downloaded from mcr.aacrjournals.org on September 26, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 1, 2018; DOI: 10.1158/1541-7786.MCR-17-0688 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Regulation and Function of H4K20me2 in DNA Repair Abstract Chromatin is a highly compact structure that must be rapidly rearranged in order for DNA repair proteins to access sites of damage and facilitate timely and efficient repair. Chromatin plasticity is achieved through multiple processes, including the post- translational modification of histone tails. In recent years, the impact of histone post- translational modification on the DNA damage response has become increasingly well recognized, and chromatin plasticity has been firmly linked to efficient DNA repair. -

Genome-Wide Analysis of HPV Integration in Human Cancers Reveals Recurrent, Focal Genomic Instability
Downloaded from genome.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability Keiko Akagi*a,b,c, Jingfeng Li*a,b,c, Tatevik R. Broutianb,d, Hesed Padilla-Nashf, Weihong Xiaob,d, Bo Jiangb,d, James W. Roccog,h, Theodoros N. Teknosi, Bhavna Kumari, Danny Wangsaf, Dandan Hea,b,c, Thomas Riedf, David E. Symer** ŧ a,b,c,d,e, Maura L. Gillison** ŧ b,d aHuman Cancer Genetics Program and bViral Oncology Program, Departments of cMolecular Virology, Immunology and Medical Genetics, dInternal Medicine and eBioinformatics, The Ohio State University Comprehensive Cancer Center, Columbus OH; fCancer Genomics Section, Center for Cancer Research, National Cancer Institute, Bethesda, MD; gCenter for Cancer Research and Department of Surgery, Massachusetts General Hospital, Boston, MA; hDepartment of Otolaryngology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA; iDepartment of Otolaryngology-Head and Neck Surgery, The Ohio State University Medical Center *These authors contributed equally to this work **These authors contributed equally to this work ŧ Corresponding authors David E. Symer, M.D., Ph.D. [email protected] Maura L. Gillison M.D., Ph.D. [email protected] Downloaded from genome.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Akagi and Li et al SUMMARY Genomic instability is a hallmark of human cancers, including the 5% caused by human papillomavirus (HPV). Here we report a striking association between HPV integration and adjacent host genomic structural variation in human cancer cell lines and primary tumors. -

The Emerging Role for Cullin 4 Family of E3 Ligases in Tumorigenesis T ⁎ ⁎ Ji Chenga,B,1, Jianping Guob,1, Brian J
BBA - Reviews on Cancer 1871 (2019) 138–159 Contents lists available at ScienceDirect BBA - Reviews on Cancer journal homepage: www.elsevier.com/locate/bbacan Review The emerging role for Cullin 4 family of E3 ligases in tumorigenesis T ⁎ ⁎ Ji Chenga,b,1, Jianping Guob,1, Brian J. Northb, Kaixiong Taoa, Pengbo Zhouc, , Wenyi Weib, a Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China b Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA c Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, 1300 York Ave., New York, NY 10065, USA ARTICLE INFO ABSTRACT Keywords: As a member of the Cullin-RING ligase family, Cullin-RING ligase 4 (CRL4) has drawn much attention due to its CRL4, Cullin 4 broad regulatory roles under physiological and pathological conditions, especially in neoplastic events. Based on E3 ligases evidence from knockout and transgenic mouse models, human clinical data, and biochemical interactions, we PROTACs summarize the distinct roles of the CRL4 E3 ligase complexes in tumorigenesis, which appears to be tissue- and Tumorigenesis context-dependent. Notably, targeting CRL4 has recently emerged as a noval anti-cancer strategy, including Targeted therapy thalidomide and its derivatives that bind to the substrate recognition receptor cereblon (CRBN), and anticancer sulfonamides that target DCAF15 to suppress the neoplastic proliferation of multiple myeloma and colorectal cancers, respectively. To this end, PROTACs have been developed as a group of engineered bi-functional che- mical glues that induce the ubiquitination-mediated degradation of substrates via recruiting E3 ligases, such as CRL4 (CRBN) and CRL2 (pVHL).