Role of Histone Methylation in Maintenance of Genome Integrity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Predicting Double-Strand DNA Breaks Using Epigenome Marks Or DNA at Kilobase Resolution
bioRxiv preprint doi: https://doi.org/10.1101/149039; this version posted June 12, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Predicting double-strand DNA breaks using epigenome marks or DNA at kilobase resolution Rapha¨elMourad1 and Olivier Cuvier1 April 10, 2017 1 Laboratoire de Biologie Mol´eculaireEucaryote (LBME), CNRS, Universit´ePaul Sabatier (UPS), 31000 Toulouse, France Abstract Double-strand breaks (DSBs) result from the attack of both DNA strands by multiple sources, includ- ing exposure to ionizing radiation or reactive oxygen species. DSBs can cause abnormal chromosomal rearrangements which are linked to cancer development, and hence represent an important issue. Recent techniques allow the genome-wide mapping of DSBs at high resolution, enabling the comprehensive study of DSB origin. However these techniques are costly and challenging. Hence we devised a computational approach to predict DSBs using the epigenomic and chromatin context, for which public data are available from the ENCODE project. We achieved excellent prediction accuracy (AUC = 0:97) at high resolution (< 1 kb), and showed that only chromatin accessibility and H3K4me1 mark were sufficient for highly accurate prediction (AUC = 0:95). We also demonstrated the better sensitivity of DSB predictions com- pared to BLESS experiments. We identified chromatin accessibility, activity and long-range contacts as best predictors. In addition, our work represents the first step toward unveiling the "cis-DNA repairing" code underlying DSBs, paving the way for future studies of cis-elements involved in DNA damage and repair. -

Application of Histone Modification-Specific Interaction Domains As an Alternative to Antibodies
Downloaded from genome.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press Method Application of histone modification-specific interaction domains as an alternative to antibodies Goran Kungulovski,1 Ina Kycia,1,4 Raluca Tamas,1 Renata Z. Jurkowska,1 Srikanth Kudithipudi,1 Chisato Henry,2 Richard Reinhardt,3 Paul Labhart,2 and Albert Jeltsch1 1Institute of Biochemistry, Stuttgart University, 70569 Stuttgart, Germany; 2Active Motif, Carlsbad, California 92008, USA; 3Max-Planck-Genomzentrum Koln,€ 50829 Koln,€ Germany Post-translational modifications (PTMs) of histones constitute a major chromatin indexing mechanism, and their proper characterization is of highest biological importance. So far, PTM-specific antibodies have been the standard reagent for studying histone PTMs despite caveats such as lot-to-lot variability of specificity and binding affinity. Herein, we suc- cessfully employed naturally occurring and engineered histone modification interacting domains for detection and identification of histone PTMs and ChIP-like enrichment of different types of chromatin. Our results demonstrate that histone interacting domains are robust and highly specific reagents that can replace or complement histone modification antibodies. These domains can be produced recombinantly in Escherichia coli at low cost and constant quality. Protein design of reading domains allows for generation of novel specificities, addition of affinity tags, and preparation of PTM binding pocket variants as matching negative controls, which is not possible with antibodies. [Supplemental material is available for this article.] The unstructured N-terminal tails of histones protrude from the yielding false negative results. When undocumented, the cross- core nucleosome and harbor complex patterns of post-translational reactivity with related or unrelated marks and the combinatorial modifications (PTMs) (Kouzarides 2007; Margueron and Reinberg effect of neighboring marks compromise the application of anti- 2010; Bannister and Kouzarides 2011; Tan et al. -

Recognition of Cancer Mutations in Histone H3K36 by Epigenetic Writers and Readers Brianna J
EPIGENETICS https://doi.org/10.1080/15592294.2018.1503491 REVIEW Recognition of cancer mutations in histone H3K36 by epigenetic writers and readers Brianna J. Kleina, Krzysztof Krajewski b, Susana Restrepoa, Peter W. Lewis c, Brian D. Strahlb, and Tatiana G. Kutateladzea aDepartment of Pharmacology, University of Colorado School of Medicine, Aurora, CO, USA; bDepartment of Biochemistry & Biophysics, The University of North Carolina School of Medicine, Chapel Hill, NC, USA; cWisconsin Institute for Discovery, University of Wisconsin, Madison, WI, USA ABSTRACT ARTICLE HISTORY Histone posttranslational modifications control the organization and function of chromatin. In Received 30 May 2018 particular, methylation of lysine 36 in histone H3 (H3K36me) has been shown to mediate gene Revised 1 July 2018 transcription, DNA repair, cell cycle regulation, and pre-mRNA splicing. Notably, mutations at or Accepted 12 July 2018 near this residue have been causally linked to the development of several human cancers. These KEYWORDS observations have helped to illuminate the role of histones themselves in disease and to clarify Histone; H3K36M; cancer; the mechanisms by which they acquire oncogenic properties. This perspective focuses on recent PTM; methylation advances in discovery and characterization of histone H3 mutations that impact H3K36 methyla- tion. We also highlight findings that the common cancer-related substitution of H3K36 to methionine (H3K36M) disturbs functions of not only H3K36me-writing enzymes but also H3K36me-specific readers. The latter case suggests that the oncogenic effects could also be linked to the inability of readers to engage H3K36M. Introduction from yeast to humans and has been shown to have a variety of functions that range from the control Histone proteins are main components of the of gene transcription and DNA repair, to cell cycle nucleosome, the fundamental building block of regulation and nutrient stress response [8]. -

Targeting the Methyltransferase SETD8 Impairs Tumor Cell Survival and Overcomes Drug Resistance Independently of P53 Status in Multiple Myeloma
bioRxiv preprint doi: https://doi.org/10.1101/776930; this version posted September 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Targeting the methyltransferase SETD8 impairs tumor cell survival and overcomes drug resistance independently of p53 status in multiple myeloma Laurie Herviou (1,2,4), Fanny Izard (3,4), Ouissem Karmous-Gadacha (2) , Claire Gourzones (1), Celine Bellanger (1), Eva Desmedt (5), Anqi Ma (6), Laure Vincent (7) , Guillaume Cartron (4,7), Karin Vanderkerken (5), Jian Jin (6), Elke De Bruyne (5), Charlotte Grimaud (3,4,8), Eric Julien (3,4,8 +) and Jérôme Moreaux (1,2,4+) (1) IGH, CNRS, Univ Montpellier, France (2) CHU Montpellier, Laboratory for Monitoring Innovative Therapies, Department of Biological Hematology, Montpellier, France (3) Institut de Recherche en Cancérologie de Montpellier (IRCM), INSERM U1194, Institut Régional du Cancer (ICM), Montpellier F-34298, France (4) University of Montpellier, Montpellier, F-34090, France (5) Department of Hematology and Immunology-Myeloma Center Brussels, Vrije Universiteit Brussel, Brussels, Belgium (6) Mount Sinai Center for Therapeutics Discovery, Departments of Pharmacological Sciences and Oncological Sciences, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, NeW York, NeW York 10029, United States. (7) CHU Montpellier, Department of Clinical Hematology, Montpellier, France (8) Centre National de la Recherche Scientifique (CNRS), F-34293, Montpellier, France + : co-last and corresponding authors ; corresponding authors: Eric Julien ([email protected]) and jérôme Moreaux ([email protected]). 1 bioRxiv preprint doi: https://doi.org/10.1101/776930; this version posted September 20, 2019. -

TRCP Promotes Cell Growth by Targeting PR-Set7/Set8 for Degradation
SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Wang, Z., X. Dai, J. Zhong, H. Inuzuka, L. Wan, X. Li, L. Wang, et al. 2015. “SCFβ-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation.” Nature Communications 6 (1): 10185. doi:10.1038/ ncomms10185. http://dx.doi.org/10.1038/ncomms10185. Published Version doi:10.1038/ncomms10185 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:23993465 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ARTICLE Received 4 May 2015 | Accepted 12 Nov 2015 | Published 15 Dec 2015 DOI: 10.1038/ncomms10185 OPEN SCFb-TRCP promotes cell growth by targeting PR-Set7/Set8 for degradation Zhiwei Wang1,2,*, Xiangpeng Dai2,*, Jiateng Zhong2,3,*, Hiroyuki Inuzuka2, Lixin Wan2, Xiaoning Li2,4, Lixia Wang1, Xiantao Ye1, Liankun Sun4, Daming Gao2,5,LeeZou6 & Wenyi Wei2 The Set8/PR-Set7/KMT5a methyltransferase plays critical roles in governing transcriptional regulation, cell cycle progression and tumorigenesis. Although CRL4Cdt2 was reported to regulate Set8 stability, deleting the PIP motif only led to partial resistance to ultraviolet- induced degradation of Set8, indicating the existence of additional E3 ligase(s) controlling Set8 stability. Furthermore, it remains largely undefined how DNA damage-induced kinase cascades trigger the timely destruction of Set8 to govern tumorigenesis. -
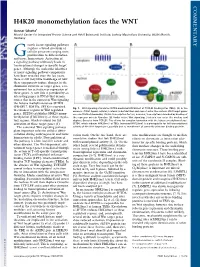
H4K20 Monomethylation Faces the WNT
COMMENTARY H4K20 monomethylation faces the WNT Gunnar Schotta1 Munich Center for Integrated Protein Science and Adolf Butenandt Institute, Ludwig Maximilians University, 80336 Munich, Germany rowth factor signaling pathways regulate a broad spectrum of G cellular processes ranging from proliferation to differentiation and tissue homeostasis. Activation of a signaling pathway ultimately leads to transcriptional changes in specific target genes. Although the molecular identities of many signaling pathway components have been revealed over the last years, there is still very little knowledge of how these components induce changes in the chromatin structure of target genes, a re- quirement for activation or repression of these genes. A new link is provided by an interesting paper in PNAS that demon- strates that in the context of Wnt signaling the histone methyltransferase SETD8 (PR-SET7, KMT5a, SET8) is recruited Fig. 1. Wnt signaling stimulates SETD8-mediated H4K20me1 at TCF/LEF binding sites (TBEs). (A)Inthe to enhancer regions of Wnt-regulated absence of Wnt ligand, cellular β-catenin is destabilized and cannot enter the nucleus. Wnt target genes genes. SETD8 establishes H4K20 mono- are constitutively bound by TCF/LEF transcription factors; however, transcription is blocked by binding of methylation (H4K20me1) at these regula- the repressor protein Groucho. (B) Under active Wnt signaling, β-catenin can enter the nucleus and tory regions, which is crucial for full displace Groucho from TCF/LEF. This allows for complex formation with the histone methyltransferase activation of these target genes (1). SETD8, which induces H4K20me1 at TBEs. Increased H4K20me1 is a prerequisite for full transcriptional The canonical Wnt signaling pathway activity of the Wnt target gene, possibly due to recruitment of currently unknown binding proteins. -

Screening for Genes That Accelerate the Epigenetic Aging Clock in Humans Reveals a Role for the H3K36 Methyltransferase NSD1 Daniel E
Martin-Herranz et al. Genome Biology (2019) 20:146 https://doi.org/10.1186/s13059-019-1753-9 RESEARCH Open Access Screening for genes that accelerate the epigenetic aging clock in humans reveals a role for the H3K36 methyltransferase NSD1 Daniel E. Martin-Herranz1,2* , Erfan Aref-Eshghi3,4, Marc Jan Bonder1,5, Thomas M. Stubbs2, Sanaa Choufani6, Rosanna Weksberg6, Oliver Stegle1,5,7, Bekim Sadikovic3,4, Wolf Reik8,9,10*† and Janet M. Thornton1*† Abstract Background: Epigenetic clocks are mathematical models that predict the biological age of an individual using DNA methylation data and have emerged in the last few years as the most accurate biomarkers of the aging process. However, little is known about the molecular mechanisms that control the rate of such clocks. Here, we have examined the human epigenetic clock in patients with a variety of developmental disorders, harboring mutations in proteins of the epigenetic machinery. Results: Using the Horvath epigenetic clock, we perform an unbiased screen for epigenetic age acceleration in the blood of these patients. We demonstrate that loss-of-function mutations in the H3K36 histone methyltransferase NSD1, which cause Sotos syndrome, substantially accelerate epigenetic aging. Furthermore, we show that the normal aging process and Sotos syndrome share methylation changes and the genomic context in which they occur. Finally, we found that the Horvath clock CpG sites are characterized by a higher Shannon methylation entropy when compared with the rest of the genome, which is dramatically decreased in Sotos syndrome patients. Conclusions: These results suggest that the H3K36 methylation machinery is a key component of the epigenetic maintenance system in humans, which controls the rate of epigenetic aging, and this role seems to be conserved in model organisms. -

Histone H4 Lysine 20 Mono-Methylation Directly Facilitates Chromatin Openness and Promotes Transcription of Housekeeping Genes
ARTICLE https://doi.org/10.1038/s41467-021-25051-2 OPEN Histone H4 lysine 20 mono-methylation directly facilitates chromatin openness and promotes transcription of housekeeping genes Muhammad Shoaib 1,8,9, Qinming Chen2,9, Xiangyan Shi 3, Nidhi Nair1, Chinmayi Prasanna 2, Renliang Yang2,4, David Walter1, Klaus S. Frederiksen 5, Hjorleifur Einarsson1, J. Peter Svensson 6, ✉ ✉ ✉ Chuan Fa Liu 2, Karl Ekwall6, Mads Lerdrup 7 , Lars Nordenskiöld 2 & Claus S. Sørensen 1 1234567890():,; Histone lysine methylations have primarily been linked to selective recruitment of reader or effector proteins that subsequently modify chromatin regions and mediate genome functions. Here, we describe a divergent role for histone H4 lysine 20 mono-methylation (H4K20me1) and demonstrate that it directly facilitates chromatin openness and accessibility by disrupting chromatin folding. Thus, accumulation of H4K20me1 demarcates highly accessible chromatin at genes, and this is maintained throughout the cell cycle. In vitro, H4K20me1-containing nucleosomal arrays with nucleosome repeat lengths (NRL) of 187 and 197 are less compact than unmethylated (H4K20me0) or trimethylated (H4K20me3) arrays. Concordantly, and in contrast to trimethylated and unmethylated tails, solid-state NMR data shows that H4K20 mono-methylation changes the H4 conformational state and leads to more dynamic histone H4-tails. Notably, the increased chromatin accessibility mediated by H4K20me1 facilitates gene expression, particularly of housekeeping genes. Altogether, we show how the methy- lation state of a single histone H4 residue operates as a focal point in chromatin structure control. While H4K20me1 directly promotes chromatin openness at highly transcribed genes, it also serves as a stepping-stone for H4K20me3-dependent chromatin compaction. -

Mechanism of CRL4 , a PCNA-Dependent E3 Ubiquitin Ligase
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase Courtney G. Havens and Johannes C. Walter1 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, USA Eukaryotic cell cycle transitions are driven by E3 ubiq- the ubiquitin is transferred from E1 to the cysteine of an uitin ligases that catalyze the ubiquitylation and destruc- ‘‘E2’’ ubiquitin-conjugating enzyme. Finally, the E2 in- tion of specific protein targets. For example, the anaphase- teracts with an ‘‘E3’’ ubiquitin ligase that also binds the promoting complex/cyclosome (APC/C) promotes the substrate. The juxtaposition of the substrate and the charged exit from mitosis via destruction of securin and mitotic E2 enzyme leads to ubiquitin transfer to the substrate. cyclins, whereas CRL1Skp2 allows entry into S phase by The specificity of ubiquitylation is encoded at the level of targeting the destruction of the cyclin-dependent kinase substrate recognition by the E3 enzymes (Ravid and (CDK) inhibitor p27. Recently, an E3 ubiquitin ligase Hochstrasser 2008); however, recently it has become called CRL4Cdt2 has been characterized, which couples clear that E2s can also contribute to processivity and proteolysis to DNA synthesis via an unusual mechanism specificity for ubiquitin chain nucleation and elongation that involves display of substrate degrons on the DNA (Jin et al. 2008; Ye and Rape 2009; Rodrigo-Brenni et al. polymerase processivity factor PCNA. Through its de- 2010; Saha et al. 2011; Wickliffe et al. 2011). Generally, struction of Cdt1, p21, and Set8, CRL4Cdt2 has emerged as the E3–substrate interaction involves the binding of a master regulator that prevents rereplication in S phase. -

Histone H3 Lysine 36 Methylation Affects Temperature-Induced Alternative Splicing and Flowering in Plants A
Pajoro et al. Genome Biology (2017) 18:102 DOI 10.1186/s13059-017-1235-x RESEARCH Open Access Histone H3 lysine 36 methylation affects temperature-induced alternative splicing and flowering in plants A. Pajoro1,2, E. Severing3,4, G. C. Angenent1,2 and R. G. H. Immink1,2* Abstract Background: Global warming severely affects flowering time and reproductive success of plants. Alternative splicing of pre-messenger RNA (mRNA) is an important mechanism underlying ambient temperature-controlled responses in plants, yet its regulation is poorly understood. An increase in temperature promotes changes in plant morphology as well as the transition from the vegetative to the reproductive phase in Arabidopsis thaliana via changes in splicing of key regulatory genes. Here we investigate whether a particular histone modification affects ambient temperature-induced alternative splicing and flowering time. Results: We use a genome-wide approach and perform RNA-sequencing (RNA-seq) analyses and histone H3 lysine 36 tri-methylation (H3K36me3) chromatin immunoprecipitation sequencing (ChIP-seq) in plants exposed to different ambient temperatures. Analysis and comparison of these datasets reveal that temperature-induced differentially spliced genes are enriched in H3K36me3. Moreover, we find that reduction of H3K36me3 deposition causes alteration in temperature-induced alternative splicing. We also show that plants with mutations in H3K36me3 writers, eraser, or readers have altered high ambient temperature-induced flowering. Conclusions: Our results show a key role for the histone mark H3K36me3 in splicing regulation and plant plasticity to fluctuating ambient temperature. Our findings open new perspectives for the breeding of crops that can better cope with environmental changes due to climate change. -

Novel Pharmacological Maps of Protein Lysine Methyltransferases: Key for Target Deorphanization Obdulia Rabal* , Andrea Castellar and Julen Oyarzabal*
Rabal et al. J Cheminform (2018) 10:32 https://doi.org/10.1186/s13321-018-0288-5 RESEARCH ARTICLE Open Access Novel pharmacological maps of protein lysine methyltransferases: key for target deorphanization Obdulia Rabal* , Andrea Castellar and Julen Oyarzabal* Abstract Epigenetic therapies are being investigated for the treatment of cancer, cognitive disorders, metabolic alterations and autoinmune diseases. Among the diferent epigenetic target families, protein lysine methyltransferases (PKMTs), are especially interesting because it is believed that their inhibition may be highly specifc at the functional level. Despite its relevance, there are currently known inhibitors against only 10 out of the 50 SET-domain containing members of the PKMT family. Accordingly, the identifcation of chemical probes for the validation of the therapeutic impact of epigenetic modulation is key. Moreover, little is known about the mechanisms that dictate their substrate specifc- ity and ligand selectivity. Consequently, it is desirable to explore novel methods to characterize the pharmacologi- cal similarity of PKMTs, going beyond classical phylogenetic relationships. Such characterization would enable the prediction of ligand of-target efects caused by lack of ligand selectivity and the repurposing of known compounds against alternative targets. This is particularly relevant in the case of orphan targets with unreported inhibitors. Here, we frst perform a systematic study of binding modes of cofactor and substrate bound ligands with all available SET domain-containing PKMTs. Protein ligand interaction fngerprints were applied to identify conserved hot spots and contact-specifc residues across subfamilies at each binding site; a relevant analysis for guiding the design of novel, selective compounds. Then, a recently described methodology (GPCR-CoINPocket) that incorporates ligand contact information into classical alignment-based comparisons was applied to the entire family of 50 SET-containing proteins to devise pharmacological similarities between them.