Using Enhanced Water to Dilute Powdered Formula Metabolic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Medications That Are Safe During Pregnancy
Medications that Are Safe during Pregnancy Women who are between four and 12 weeks pregnant may safely take the following over-the-counter medications. Follow all directions on the container for adult dosage/use. ~r---------~ I Problem Over the Counter ICall Your Care Provider for: c __ ; Morning sickness Vitamin 86: take 50 mg/day to start; if not Persistent vomiting; weight loss or helpful, increase by 50 mg 2 to 4 times/day inability to tolerate fluids for 24 hours until you reach a total of 200 mg/day. Do not take more than 200 mg each day. I Increase fluids. i I Mild headaches / general Try comfort measures. Severe and/or persistent headaches I aches & pains Acetominophen (Tylenol) - ~ ~""~'" I Nasal congestion due to a IOcean Mist nasal spray I cold, sinusitis or allergies I I -, Women who are more than 12 weeks pregnant may safely take the following over-the-counter medications. Follow all directions on the container for adult dosage/use. I Problem lover the Counter 1Call Your Care Provider for: '" '''''Moo "0'''"0'''''''''''" , Nasal congestion due to a Sudafed, Afrin nasal spray, Ocean Mist cold, sinusitis or allergies nasal spray I I i Cough due to minor throat Robitussin (or other brand of Guaifenesin), Persistent cough i irritation Robitussin DM or non-alcohol cough syrup (not to exceed 1 ! week's use) i I Nasal congestion and coUgh Triaminic DM (or other brand of alcohol- free and antihistamine-free decongestant I and antitussive) I I I Sore throat Alcohol-free lozenges, such as Severe or persistent sore throat Chloraseptic -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

General Instructions for Infant and Child Care
Name _______________________________________________ Birth Date ____________________________________________ GENERAL INSTRUCTIONS FOR INFANT AND CHILD CARE GUIDELINES FOR HEALTH EVALUATION VISITS Richmond Pediatrics Pediatric & Adolescent Medicine . for over 50 years 357 N.W. Richmond Beach Road Shoreline, Washington 98177 (206) 546-2421 (206) 546-8436 – Fax www.Richmond-Pediatrics.com Age Immunization Date Given Immunizations >9 Years Old Birth Hepatitis B Immunization Date Given Hepatitis B MCV4 (Meningitis) #1 DtaP MCV4 (Meningitis) #2 IPV (Polio) 2 Months TdaP Hib (Meningitis) HPV #1 PCV13 (Pneumonia) Rotavirus HPV #2 DtaP HPV #3 IPV (Polio) 4 Months Hib (Meningitis) We also recommend a yearly influenza immunization. PCV13 (Pneumonia) Rotavirus Hepatitis B DtaP IPV (Polio) 6 Months Hib (Meningitis) PCV13 (Pneumonia) Rotavirus MMR VZV (Chickenpox) DtaP 12 -18 Hib (Meningitis) Months PCV13 (Pneumonia) Hepatitis A #1 Hepatitis A #2 18mos-4yrs PCV13 booster DtaP IPV 5 Years MMR VZV (Chickenpox) Influenza #1 6mos-5 yrs Influenza #2 TABLE OF CONTENTS Infant Care Page Breast Feeding ..............................................................6 Diarrhea .......................................................................20 Formula .........................................................................8 Dehydration .................................................................20 Feeding Schedule .........................................................9 Fever ...........................................................................22 -
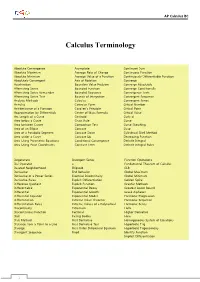
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -
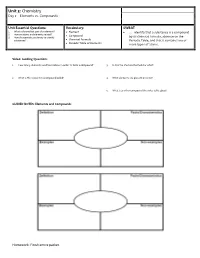
Unit 3: Chemistry Day 2 – Elements Vs
Unit 3: Chemistry Day 2 – Elements vs. Compounds Unit Essential Questions: Vocabulary: SWBAT 1. What is the smallest part of an element? Element … identify that a substance is a compound 2. How are atoms and elements related? Compound 3. How do scientists use density to identify by its chemical formula, absence on the substances? Chemical Formula Periodic Table, and that it contains two or Periodic Table of Elements more types of atoms. Video: Guiding Questions 1. How many elements need to combine in order to form a compound? 3. H2O is the chemical formula for what? 2. What is the recipe for a compound called? 4. What elements are present in water? 5. What is another compound the video talks about? GUIDED NOTES: Elements and Compounds Homework: Finish entire packet. STOP AND JOT: What is the difference between an element and a compound? Chemical Formulas Compounds are represented by _________________________________ _____________________________________. Each capital letter represents _______________ element. ____________________________________ represent the number of atoms of each element. Examples: H2O Si3N4 Number of elements: _____________________ Number of elements: _____________________ Number of atoms for each element: Number of atoms for each element: ______________________ ______________________ ______________________ ________________________ How many elements are in How many atoms of each How many elements are in How many atoms of each the compound H3PO4? element is present in the the compound C2H5OH? element is present in the compound, HNO3? compound, C2H4O2? Three ways to identify a Compound: 1. Made of more than one _________________________. 2. Not located on the _____________________________ __________________________ _________________ ________________________________. 3. Represented by a ____________________________________ ____________________________________. -

What to Do When Your Child Has the “Tummy Flu”?
What to do when your child has the “Tummy flu”? How can I take care of my child? Vomiting: 1. Diet for breast-fed babies The key to treatment is providing breast milk in smaller amounts than usual. If your baby vomits once, make no changes. If your baby vomits twice, continue breast feeding but nurse on only one side for 10 minutes every 1 to 2 hours. If your baby vomits 3 or more times, nurse for 4 to 5 minutes every 30 to 60 minutes. As soon as 8 have passed without vomiting, return to normal nursing on both sides. Pedialyte is rarely needed for breast-fed babies. If vomiting continues, switch to Pedialyte for 4 hours. Spoon or syringe feed 1 to 2 teaspoons (5 to 10 ml) of Pedialyte every 5 minutes. If your baby is urinating less than normal, you can offer the baby an electrolyte solution between breast-feedings for a short time (6 to 24 hours). 2. Diet for formula-fed babies For infants less than 1 year old, always use an oral electrolyte solution (such as Pedialyte). Spoon or syringe feed your baby 1 teaspoon (5 ml) every 5 minutes. Until you get some Pedialyte, give formula by teaspoon in the same way. 3. If the child is vomiting offer small amount of clear fluids for 8 hours (no solid food) Offer clear fluids (no milk) in small amounts until 8 hours have passed without vomiting. Preferably Pedialyte and/or other oral rehydration formulas. Pedialyte popsicles, Jell-O are other ways to get fluids into the child. -

Approach to Pediatric Vomiting.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Approach to Pediatric Vomiting.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Developed by Erin Boschee and Dr. Melanie Lewis for PedsCases.com. August 25, 2014. Approach to Pediatric Vomiting (Part 1) Introduction Hi, Everyone! My name is Erin Boschee and I’m a medical student at the University of Alberta. This podcast was reviewed by Dr. Melanie Lewis, a General Pediatrician and Associate Professor at the University of Alberta and Stollery Children’s Hospital in Edmonton, Alberta, Canada. This is the first in a series of two podcasts discussing an approach to pediatric vomiting. We will focus on the following learning objectives: 1) Create a differential diagnosis for pediatric vomiting. 2) Highlight the key causes of vomiting specific to the newborn and pediatric population. 3) Develop a clinical approach to pediatric vomiting through history taking, physical exam and investigations. Case Example Let’s start with a case example that we will revisit at the end of the podcasts. You are called to assess a 3-week old male infant for recurrent vomiting and ‘feeding difficulties.’ The ER physician tells you that the mother brought the baby in stating that he started vomiting with every feed since around two weeks of age. In the last three days he has become progressively more sleepy and lethargic. She brought him in this afternoon because he vomited so forcefully that it sprayed her in the face. -

E.W. Dijkstra Archive: on the Cruelty of Really Teaching Computing Science
On the cruelty of really teaching computing science Edsger W. Dijkstra. (EWD1036) http://www.cs.utexas.edu/users/EWD/ewd10xx/EWD1036.PDF The second part of this talk pursues some of the scientific and educational consequences of the assumption that computers represent a radical novelty. In order to give this assumption clear contents, we have to be much more precise as to what we mean in this context by the adjective "radical". We shall do so in the first part of this talk, in which we shall furthermore supply evidence in support of our assumption. The usual way in which we plan today for tomorrow is in yesterday’s vocabulary. We do so, because we try to get away with the concepts we are familiar with and that have acquired their meanings in our past experience. Of course, the words and the concepts don’t quite fit because our future differs from our past, but then we stretch them a little bit. Linguists are quite familiar with the phenomenon that the meanings of words evolve over time, but also know that this is a slow and gradual process. It is the most common way of trying to cope with novelty: by means of metaphors and analogies we try to link the new to the old, the novel to the familiar. Under sufficiently slow and gradual change, it works reasonably well; in the case of a sharp discontinuity, however, the method breaks down: though we may glorify it with the name "common sense", our past experience is no longer relevant, the analogies become too shallow, and the metaphors become more misleading than illuminating. -
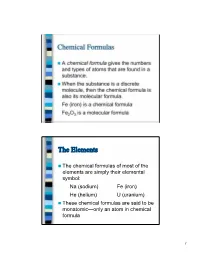
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom. -

Caring for Raccoons.Pdf
CARECARE OFOF ORPHANEDORPHANED RACOONSRACOONS Compiled by Laurel A. Beechey [email protected] 94 North St. W. Tillsonburg, On. Canada N4G1C3 519-842-9416 CARE OF ORPHANED RACCOONS For Wildlife Guardians of Ontario This information is from a conglomerate of sources including IWRA & experiences of several Canadian Wildlife Rehabbers. Index Infants Page 3 Keep Warm Page 3 Examine Page 3 Dehydrated Page 3 Re-hydrate Page 3 Formula Page 4 Nipples Page 4 Weaning Page 4 Post Weaning Page 4 Wild Diet Page 4 Store bought foods Page 4 Poop & Pee Page 5 Housing Page 5 Roundworm Page 6 Quarantine Page 7 Diseases Page 6 Lung Infections Page 7 Coccidia Page 7 Toxemia Page 7 Release Page 7 Fast Release Page 7 Post Release Page 7 Growth Chart Page 8 Feeding Chart Page 9 2 INFANT RACCOON Keep Warm: Put the baby/babies in a box or cage with lots of rags and place a portion of the box/cage on a heating pad on low, leaving a portion unheated to allow the baby to move from the heat if necessary. Hot water in a jar, wrapped in a towel or a ‘hot water bottle’ can be used but the temperature must be monitored. If eyes are closed they will not climb but if eyes are open a secure lid is recommended so they don’t climb out. Find a wildlife rehabilitator in your area and get the animal to them as soon as possible. Can't locate one yet? Do the following and get the baby to a rehabber as soon as possible. -

Common Equations Used in Chemistry Equation For
Common Equations Used in Chemistry m Equation for density: d= v 5 Converting ˚F to ˚C: ˚C = (˚F - 32) x 9 9 Converting ˚C to ˚F: ˚F = ˚C x 5 + 32 Converting ˚C to K: K = (˚C + 273.15) n x molar mass of element Percent composition of an element = molar mass of compound x 100% - where n = the number of moles of the element in one mole of the compound actual yield % yield = theoretical yield x 100% moles of solute molarity (M) = liters of solution Dilution of Solution: MiVi = MfV f Boyle’s law - Constant T and n: PV = k Boyle’s law - For calculating changes in pressure or volume: P1V1 = P2V 2 V Charles’ law - Constant P and n: T = k V1 V 2 Charles’ law - For calculating temperature or volume changes: = T1 T2 Avogadro’s law - Constant P and T: V = kn Ideal Gas equation: PV = nRT Calculation of changes in pressure, temperature, or volume of gas when n is constant: P1V1 P2V 2 = T1 T2 PM Calculation of density or molar mass of gas: d = RT Dalton’s law of partial pressures - for calculating partial pressures: Pi = XiPT 3RT 0.5 Root-mean-square speed of gas molecules: urms = ( M ) Van der waals equation; for calculating the pressure of a nonideal gas: an 2 (P + ) (V - nb) = nRT V 2 Definition of heat capacity, where s is specific heat: C = ms Calculation of heat change in terms of specific heat : q = msDt Calculation of heat change in terms of heat capacity: q = CDt q1q2 Electrical force: F = k el r2 q1q2 Potential energy: V = k r Calculation of standard enthalpy of reaction: DH˚rxn = å nDH˚f (products) - å mDH˚f (reactants) [where n and m are coefficients in equation] Mathematical statement of the first law of thermodynamics: DE = q + w Work done in gas expansion or compression: w = - PDV Definition of enthalpy: H = E + PV Enthalpy (or energy) change for a constant-pressure process: DH = DE +PDV Enthalpy (or energy) change for a constant-pressure process: DE = DH - RTDn, where n is the change in the number of moles of gas.