Unit 3: Chemistry Day 2 – Elements Vs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -
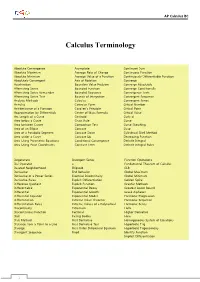
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

E.W. Dijkstra Archive: on the Cruelty of Really Teaching Computing Science
On the cruelty of really teaching computing science Edsger W. Dijkstra. (EWD1036) http://www.cs.utexas.edu/users/EWD/ewd10xx/EWD1036.PDF The second part of this talk pursues some of the scientific and educational consequences of the assumption that computers represent a radical novelty. In order to give this assumption clear contents, we have to be much more precise as to what we mean in this context by the adjective "radical". We shall do so in the first part of this talk, in which we shall furthermore supply evidence in support of our assumption. The usual way in which we plan today for tomorrow is in yesterday’s vocabulary. We do so, because we try to get away with the concepts we are familiar with and that have acquired their meanings in our past experience. Of course, the words and the concepts don’t quite fit because our future differs from our past, but then we stretch them a little bit. Linguists are quite familiar with the phenomenon that the meanings of words evolve over time, but also know that this is a slow and gradual process. It is the most common way of trying to cope with novelty: by means of metaphors and analogies we try to link the new to the old, the novel to the familiar. Under sufficiently slow and gradual change, it works reasonably well; in the case of a sharp discontinuity, however, the method breaks down: though we may glorify it with the name "common sense", our past experience is no longer relevant, the analogies become too shallow, and the metaphors become more misleading than illuminating. -
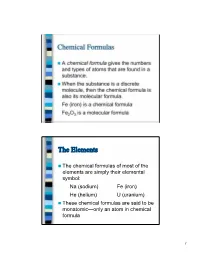
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom. -

Common Equations Used in Chemistry Equation For
Common Equations Used in Chemistry m Equation for density: d= v 5 Converting ˚F to ˚C: ˚C = (˚F - 32) x 9 9 Converting ˚C to ˚F: ˚F = ˚C x 5 + 32 Converting ˚C to K: K = (˚C + 273.15) n x molar mass of element Percent composition of an element = molar mass of compound x 100% - where n = the number of moles of the element in one mole of the compound actual yield % yield = theoretical yield x 100% moles of solute molarity (M) = liters of solution Dilution of Solution: MiVi = MfV f Boyle’s law - Constant T and n: PV = k Boyle’s law - For calculating changes in pressure or volume: P1V1 = P2V 2 V Charles’ law - Constant P and n: T = k V1 V 2 Charles’ law - For calculating temperature or volume changes: = T1 T2 Avogadro’s law - Constant P and T: V = kn Ideal Gas equation: PV = nRT Calculation of changes in pressure, temperature, or volume of gas when n is constant: P1V1 P2V 2 = T1 T2 PM Calculation of density or molar mass of gas: d = RT Dalton’s law of partial pressures - for calculating partial pressures: Pi = XiPT 3RT 0.5 Root-mean-square speed of gas molecules: urms = ( M ) Van der waals equation; for calculating the pressure of a nonideal gas: an 2 (P + ) (V - nb) = nRT V 2 Definition of heat capacity, where s is specific heat: C = ms Calculation of heat change in terms of specific heat : q = msDt Calculation of heat change in terms of heat capacity: q = CDt q1q2 Electrical force: F = k el r2 q1q2 Potential energy: V = k r Calculation of standard enthalpy of reaction: DH˚rxn = å nDH˚f (products) - å mDH˚f (reactants) [where n and m are coefficients in equation] Mathematical statement of the first law of thermodynamics: DE = q + w Work done in gas expansion or compression: w = - PDV Definition of enthalpy: H = E + PV Enthalpy (or energy) change for a constant-pressure process: DH = DE +PDV Enthalpy (or energy) change for a constant-pressure process: DE = DH - RTDn, where n is the change in the number of moles of gas. -

Ethyl Alcohol
Right to Know Hazardous Substance Fact Sheet Common Name: ETHYL ALCOHOL Synonyms: Alcohol; Methylcarbinol CAS Number: 64-17-5 Chemical Name: Ethanol RTK Substance Number: 0844 Date: March 2011 Revision: March 2016 DOT Number: UN 1170 Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Ethyl Alcohol is a clear, colorless liquid with a wine-like odor. Hazard Summary It is used in alcoholic beverages, as a solvent, and in making Hazard Rating NJDHSS NFPA other chemicals. HEALTH - 2 FLAMMABILITY - 3 ODOR THRESHOLD = 84 ppm REACTIVITY - 0 Odor thresholds vary greatly. Do not rely on odor alone to determine potentially hazardous exposures. FLAMMABLE POISONOUS GASES ARE PRODUCED IN FIRE CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; 4=severe Reasons for Citation Ethyl Alcohol can affect you when inhaled and by passing Ethyl Alcohol is on the Right to Know Hazardous through the skin. Substance List because it is cited by OSHA, ACGIH, DOT, High concentrations may damage the fetus. NIOSH, IARC, NFPA and EPA. Contact can irritate the skin and eyes. Prolonged or repeated This chemical is on the Special Health Hazard Substance exposure can cause drying and cracking of the skin with List. peeling, redness and itching. Inhaling Ethyl Alcohol can irritate the nose, throat and lungs. Exposure to Ethyl Alcohol can cause headache, drowsiness, nausea and vomiting, and unconsciousness. It can also affect concentration and vision. SEE GLOSSARY ON PAGE 5. Repeated high exposure may affect the liver and the nervous system. Ethyl Alcohol is a FLAMMABLE LIQUID and a FIRST AID DANGEROUS FIRE HAZARD. -

Edsger Wybe Dijkstra
In Memoriam Edsger Wybe Dijkstra (1930–2002) Contents 0 Early Years 2 1 Mathematical Center, Amsterdam 3 2 Eindhoven University of Technology 6 3 Burroughs Corporation 8 4 Austin and The University of Texas 9 5 Awards and Honors 12 6 Written Works 13 7 Prophet and Sage 14 8 Some Memorable Quotations 16 Edsger Wybe Dijkstra, Professor Emeritus in the Computer Sciences Department at the University of Texas at Austin, died of cancer on 6 August 2002 at his home in Nuenen, the Netherlands. Dijkstra was a towering figure whose scientific contributions permeate major domains of computer science. He was a thinker, scholar, and teacher in renaissance style: working alone or in a close-knit group on problems of long-term importance, writing down his thoughts with exquisite precision, educating students to appreciate the nature of scientific research, and publicly chastising powerful individuals and institutions when he felt scientific integrity was being compromised for economic or political ends. In the words of Professor Sir Tony Hoare, FRS, delivered by him at Dijkstra’s funeral: Edsger is widely recognized as a man who has thought deeply about many deep questions; and among the deepest questions is that of traditional moral philosophy: How is it that a person should live their life? Edsger found his answer to this question early in his life: He decided he would live as an academic scientist, conducting research into a new branch of science, the science of computing. He would lay the foundations that would establish computing as a rigorous scientific discipline; and in his research and in his teaching and in his writing, he would pursue perfection to the exclusion of all other concerns. -

Ethyl Alcohol
From the Toxipedia website in original form. Last updated by Toxipedia in 2014. Ethyl Alcohol Also see our longer article, A Small Dose of Alcohol. Just the facts Physical Information Name: Ethyl Alcohol Molecular Formula: CH 3 CH 2 OH Use: solvent, intoxicant Source: home, industry, stores, and alcoholic beverages Recommended daily intake: not essential Absorption: readily absorbed by intestine, food will delay absorption Sensitive individuals: fetus (Fetal Alcohol Spectrum Disorder (FASD)) Toxicity/symptoms: developing nervous system very sensitive to low levels of exposure; kids - lowered IQ, learning and behavioral problems; adults - memory loss, inebriation, liver disease, cancer Regulatory facts: government agencies recommend women not consume alcohol during pregnancy; blood alcohol regulated by local governments when operating a motor vehicle Recommendations: do not consume alcohol during pregnancy, otherwise limit consumption and do not drive a motor vehicle after drinking 1 Chemical Structure History and Introduction Alcohol has been widely consumed for millenia for numerous reasons: part of a standard diet, for medicinal reasons, for its relaxant and euphoric effects, or for recreational purposes. The discovery of the distillation process during the 12th century made it possible to make drinks with higher alcohol content than can be achieved by the fermentation process alone (#MedLine Plus). It is from this process that alcohol got its name from Paracelsus, meaning "finely divided" in Greek which refers to distillation. It is believed that fermented beverages may date back to over 9,000 years ago, and the production and consumption of wine are mentioned several times in the Bible. Ethanol is a psychoactive drug with a depressant effects and is widely regulated. -

6.2 Properties of Logarithms 437
6.2 Properties of Logarithms 437 6.2 Properties of Logarithms In Section 6.1, we introduced the logarithmic functions as inverses of exponential functions and discussed a few of their functional properties from that perspective. In this section, we explore the algebraic properties of logarithms. Historically, these have played a huge role in the scientific development of our society since, among other things, they were used to develop analog computing devices called slide rules which enabled scientists and engineers to perform accurate calculations leading to such things as space travel and the moon landing. As we shall see shortly, logs inherit analogs of all of the properties of exponents you learned in Elementary and Intermediate Algebra. We first extract two properties from Theorem 6.2 to remind us of the definition of a logarithm as the inverse of an exponential function. Theorem 6.3. (Inverse Properties of Exponential and Log Functions) Let b > 0, b =6 1. a • b = c if and only if logb(c) = a x log (x) • logb (b ) = x for all x and b b = x for all x > 0 Next, we spell out what it means for exponential and logarithmic functions to be one-to-one. Theorem 6.4. (One-to-one Properties of Exponential and Log Functions) Let f(x) = x b and g(x) = logb(x) where b > 0, b =6 1. Then f and g are one-to-one. In other words: • bu = bw if and only if u = w for all real numbers u and w. • logb(u) = logb(w) if and only if u = w for all real numbers u > 0, w > 0. -
Problems in Elementary Mathematics
PROBLEMS IN ELEMENTARY MATHEMATICS MIR PUBLISHERS MOSCOW 3AAA 4l9 B. B..NACKIIA, IIO 3JIEMEHTAPHOf1 A.B OBCRHHHKOB, A.H. TYJIAAKOB. MATEMATHKE M. H WABYHHH H3.1 ATE.BbCTBO 4HAYKA. MOCKBA V. LIDSKY, PROBLEMS L. OVSYANNIKOV, A. TULAIKOV, M. SHABUNIN IN ELEMENTARY MATHEMATICS MIR PUBLISHERSI MOSCOW UDC 511+512+513+514(0.75.4)=20 Translated frori the Russian by V. VOLOSOV First published 1973 Revised from the 1970 Russian edition Ha axaau1cxoet R3btKe Q English translation, Mir Publishers, 1973 0223 - 374 041(01) - 73 CONTENTS ProblemsSolutions Algebra 1. Arithmetic and Geometric Progressions (1-23) 7 87 2. AlgebraicEquations and Systems ofEquations (24-95) 10 95 3. Algebraic Inequalities (96-123) . 20 134 4. Logarithmic and ExponentialEquations,Identities and Inequalities (124-169) . 24 142 5. Combinatorial Analysis and Newton's Binomial Theorem (170-188) . 29 157 6. Problems in Forming Equations (189-228) . 32 162 7. Miscellaneous Problems (229-291) ....... .. .. 38 180 Geometry A. Plane Geometry 1. Computation Problems (292-324).. .......... 47 202 2. Construction Problems (325.338) . ....... 51 217 3. Proof Problems (339-408) . .... 52 223 4. Loci of Points (409-420). ... ... 59 254 5. The Greatest and Least Values (421-430).. ...... 61 260 B. Solid Geometry 1. Computation Problems (431-500) . ........... 62 266 2. Proof Problems (501-523) . ... .. 70 309 3. Loci of Points (524-530) . .... ... 72 322 4. The Greatest and Least Values (531-532)..... ... 72 325 Trigonometry 1. Transforming Expressions Containing Trigonometric Func- tions (533-554) . 74 327 2. Trigonometric Equations and Systems of Equations (555-618) 77 333 3. Inverse Trigonometric Functions (619628) . .. 82 363 4. -

Ethyl Alcohol
ETHYL ALCOHOL Prepared at the 46th JECFA (1996), published in FNP 52 Add 4 (1996) superseding specifications prepared at the 29th JECFA (1985), published in FNP 34 (1986. An ADI limited by GMP was established at 14th JECFA (1970)) SYNONYMS Alcohol, ethanol DEFINITION Contains usually about 95% v/v (i.e. with about 5% v/v water) C2H6O; other grades with a different water content may be used depending on the technological requirements; these specifications apply only to undenaturated ethyl alcohol. Chemical names Ethanol C.A.S. number 64-17-5 Chemical formula C2H6O Structural formula Formula weight 46.07 Assay Not less than 94.9% v/v DESCRIPTION Clear colourless mobile liquid with a mild characteristic odour; flammable FUNCTIONAL USES Extraction solvent, carrier solvent, flavouring agent (see "Flavouring agents" monograph JECFA no. 41) CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Soluble in water Refractive index (Vol. 4) n (20, D): 1.364 Boiling point (Vol. 4) 78º Infrared absorption The infrared spectrum of the sample corresponds with the reference infrared spectrum below PURITY Solubility Transfer 50 ml of the sample to a 100 ml glass-stoppered graduated cylinder, dilute to 100 ml with water, and mix. Place the cylinder in a water bath maintained at 10º, and allow to stand for 30 min. No haze or turbidity develops. Non-volatile residue Not more than 2 mg/100 ml (Vol. 4) Acidity Not more than 0.005% w/v as acetic acid Transfer 10 ml of the sample to a glass-stoppered flask containing 25 ml of water, add 0.5 ml of phenolphthalein TS.