5Th Grade Lesson Plan Lego Molecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Inorganic Chemistry
General Inorganic Chemistry Pre DP Chemistry Period 1 • Teacher: Annika Nyberg • [email protected] • Urgent messages via Wilma! Klicka här för att ändra format på underrubrik i bakgrunden • Course book: • CliffsNotes: Chemistry Quick Review http://www.chem1.com/acad/webtext/virtualtextbook.html Content • Introduction • The Structure of Matter (Chapter 1) • The Atom (Chapter 2 and 3) • Chemical bonding (Chapter 5) • The Mole (Chapter 2) • Solutions (Chapter 9) • Acids and bases (Chapter 10) • Quiz • Revision • EXAM 9.00-11.45 Assessment Exam: 80 % Quiz: 20% + practical work, activity and absences 1. Chemistry: a Science for the twenty-first century ● Chemistry has ancient roots, but is now a modern and active, evolving science. ● Chemistry is often called the central science, because a basic knowledge of chemistry is essential for students in biology, physics, geology and many other subjects. https://www.youtube.com/watch?v=tTlnrhiadnI ● Chemical research and development has provided us with new substances with specific properties. These substances have improved the quality of our lives. Health and medicine vaccines sanitation systems antibiotics anesthesia and all other drugs Energy new alternative energy sources (e.g. solar energy to electric energy, nuclear fission) electric cars with long lasting batteries Environment greenhouse gases acid rain and smog Materials and Technology ● polymers (rubber and nylon), ceramics (cookware), liquid crystals (electronic displays), adhesives (Post-It notes), coatings (latex-paint), silicon chips (computers) Food and Agriculture substances for biotechnology ● The purpose of this course is to make you understand how chemists see the world. ● In other words, if you see one thing (in the macroscopic world) you think another (visualize the particles and events in the microscopic world). -

Basic Chemistry
CH 2- THE CHEMISTRY OF LIFE Atoms . The study of chemistry begins with the basic unit of matter, the atom. The Greek philosopher Democritus called the smallest fragment of matter the atom, from the Greek word atomos. Atoms (cont.) . Placed side by side, 100 million atoms would make a row only about 1 centimeter long. Atoms contain subatomic particles that are even smaller. Atoms (cont.) . What three subatomic particles make up atoms? Atoms (cont.) . The subatomic particles that make up atoms are . protons . neutrons . electrons Atoms (cont.) . Smallest property of an element that still has the properties of that element . “The building blocks of matter” . Atoms are made of smaller (subatomic) particles arranged in a particular way . p+ (proton) . n° (neutron) . e- (electron) Atoms (cont.) . Protons and neutrons have about the same mass. Protons are positively charged particles (+). Neutrons carry no charge (◦). Strong forces bind protons and neutrons together to form the nucleus, which is at the center of the atom. Atoms (cont.) . The electron is a negatively charged particle (−) with 1/1840 the mass of a proton. Electrons are in constant motion in the space surrounding the nucleus (e- cloud). Atoms (cont.) . The subatomic particles in a helium atom. Atoms (cont.) • Electrons are attracted to the positively charged nucleus but remain outside the nucleus because of the energy of their motion. • Because atoms have equal numbers of electrons and protons, and because these subatomic particles have equal but opposite charges, atoms are neutral. Atoms (cont.) . Atomic number- # of p+ AND electrons in an atom . Mass number- total # of p+ + n° in an atom . -
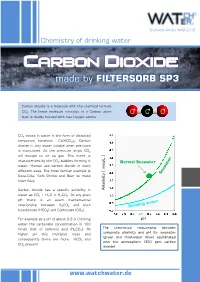
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

All About the Chemical Bonds and Compounds
All about the Chemical Bonds and Compounds Video Transcript Almost everything we see or touch in daily life – such as the food we eat, the water we drink, the air we breathe, and so on – is the result of chemical bonding. In other words, the world around us is generally not composed of isolated atoms. Instead, atoms bond to one another to form molecules and hence chemical compounds, which make up the world around us. Chemical bonding is the physical process that causes atoms and molecules to be attracted to each other and held together in more stable chemical compounds. There are three primary types of chemical bonds and compounds: Ionic bonds, covalent bonds, and metallic bonds. With ionic bonds, electrons are exchanged or transferred between atoms. They exist in ionic compounds. Generally, they’re a metal and a nonmetal – sodium chloride, magnesium oxide, etc. With covalent bonds, electrons are shared among the atoms. They exist in covalent and molecular compounds. Generally, they are nonmetals – carbon dioxide, dihydrogen monoxide, etc. With metallic bonds, a pool of electrons roam freely across entire molecule. They exist in metallic compounds. Generally, they’re metals and alloys – copper, gold, etc. A chemical compound is a group of two or more different atoms that are attracted to each other. Compounds can be divided into ionic compounds, covalent compounds, and metallic compounds. This table lists some key properties of each. Be aware that it is the valence electrons (those in the outermost level) that are involved in bonding. Atoms try to fill their outer energy levels because it’s energetically favorable for atoms to be in that configuration and it makes them stable. -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Chapter 10 – Chemical Reactions Notes
Chapter 8 – Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if a chemical reaction has occurred (recap): Temperature changes that can’t be accounted for. o Exothermic reactions give off energy (as in fire). o Endothermic reactions absorb energy (as in a cold pack). Spontaneous color change. o This happens when things rust, when they rot, and when they burn. Appearance of a solid when two liquids are mixed. o This solid is called a precipitate. Formation of a gas / bubbling, as when vinegar and baking soda are mixed. Overall, the most important thing to remember is that a chemical reaction produces a whole new chemical compound. Just changing the way that something looks (breaking, melting, dissolving, etc) isn’t enough to qualify something as a chemical reaction! Balancing Equations Notes: Things to keep in mind when looking at the recipes for chemical reactions: 1) The stuff before the arrow is referred to as the “reactants” or “reagents”, and the stuff after the arrow is called the “products.” 2) The number of atoms of each element is the same on both sides of the arrow. Even though there may be different numbers of molecules, the number of atoms of each element needs to remain the same to obey the law of conservation of mass. 3) The numbers in front of the formulas tell you how many molecules or moles of each chemical are involved in the reaction. 4) Equations are nothing more than chemical recipes. -

1 5. Chemical Bonding
5. Chemical Bonding: The Covalent Bond Model 5.1 The Covalent Bond Model Almost all chemical substances are found as aggregates of atoms in the form of molecules and ions produced through the reactions of various atoms of elements except the noble-gas elements which are stable mono-atomic gases. Chemical bond is a term that describes the attractive force that is holding the atoms of the same or different kind of atoms in forming a molecule or ionic solid that has more stability than the individual atoms. Depending on the kinds of atoms participating in the interaction there seem to be three types of bonding: Gaining or Losing Electrons: Ionic bonding: Formed between many ions formed by metal and nonmetallic elements. Sharing Electrons: Covalent bonding: sharing of electrons between two atoms of non-metals. Metallic Bonding: sharing of electrons between many atoms of metals. Ionic Compounds Covalent Compounds Metallic Compounds 1. Metal and non-meal Non-metal and non-meal Metal of one type or, element combinations. elements combinations. combinations of two or metal elements combinations. 2. High melting brittle Gases, liquids, or waxy, low Conducting, high melting, crystalline solids. melting soft solids. malleable, ductile crystalline solids. 3. Do not conduct as a solid Do not conduct electricity at Conduct electricity at solid but conducts electricity any state. and molten states. when molten. 4. Dissolved in water produce Most are soluble in non-polar Insoluble in any type of conducting solutions solvents and few in water. solvents. (electrolytes) and few These solutions are non- are soluble in non-polar conducting (non- solvents. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

Element Symbol: Pb Atomic Number: 82
LEAD Element Symbol: Pb Atomic Number: 82 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au LEAD Element symbol: Pb Atomic number: 82 Lead has atomic symbol Pb and atomic number 82. It is located towards the bottom of the periodic table, and is the heaviest element of the carbon (C) family. The chemical symbol for lead is an abbreviation from the Latin word plumbum, the same root word as plumbing, plumber and plumb-bob still commonly used today. Two different interpretations are associated with the word plumbum, namely “soft metals” and “waterworks”. No-one knows who discovered lead, the metal having been known since ancient times. Archaeological pieces from Egypt and Turkey have been found dating to around 5000 BC. Lead is also mentioned in the book of Exodus in the Bible. The Hanging Gardens of Babylon were floored with soldered sheets of lead, while there is evidence to suggest the Chinese were manufacturing lead around 3000 BC. Some reports suggest that lead was probably one of the first metals to be produced by man, and this is likely so given its worldwide occurrence and its relative ease of extraction. In alchemy, lead was considered the oldest metal and was associated with the planet Saturn. Lead makes up ~0.0013% of the earth’s crust. It is rare that metallic lead occurs naturally. Lead is typically associated with zinc, copper and silver bearing ores and is processed and extracted along with these metals. When freshly cut pure lead is a bluish-white shiny metal that readily oxidises in air to the grey metal that is more typically known.