Ambient Water Quality Criteria for Polycyclic Aromatic Hydrocarbons (Pahs)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
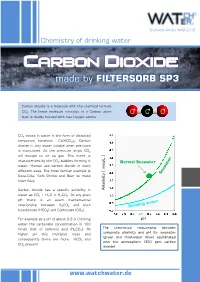
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

Polycyclic Aromatic Hydrocarbon Migration from Creosote-Treated Railway Ties Into Ballast and Adjacent Wetlands
United States In cooperation Department of with the Agriculture Polycyclic Aromatic United States Forest Service Department of Transportation Hydrocarbon Migration Forest Products Federal Laboratory Highway Administration From Creosote-Treated Research Paper FPL−RP−617 Railway Ties Into Ballast and Adjacent Wetlands Kenneth M. Brooks Abstract from the weathered ties at this time. No significant PAH loss was observed from ties during the second summer. A Occasionally, creosote-treated railroad ties need to be small portion of PAH appeared to move vertically down into replaced, sometimes in sensitive environments such as the ballast to approximately 60 cm. Small amounts of PAH wetlands. To help determine if this is detrimental to the may have migrated from the ballast into adjacent wetlands surrounding environment, more information is needed on during the second summer, but these amounts were not the extent and pattern of creosote, or more specifically poly- statistically significant. These results suggest that it is rea- cyclic aromatic hydrocarbon (PAH), migration from railroad sonable to expect a detectable migration of creosote-derived ties and what effects this would have on the surrounding PAH from newly treated railway ties into supporting ballast environment. This study is a report on PAH level testing during their first exposure to hot summer weather. The PAH done in a simulated wetland mesocosm. Both newly treated rapidly disappeared from the ballast during the fall and and weathered creosote-treated railroad ties were placed in winter following this initial loss. Then statistically insignifi- the simulated wetland. As a control, untreated ties were also cant vertical and horizontal migration of these PAH suggests placed in the mesocosm. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Safeguards Benzopyrene Contamination in Korean
SAFEGUARDS SGS CONSUMER TESTING SERVICES FOOD NO. 187/12 NOV 2012 BENZOPYRENE CONTAMINATION IN KOREAN INSTANT NOODLE On 25 Oct 2012, Korea Food and Drug Administration (KFDA) recalled six instant noodle products from the nation’s largest ramen maker in South Korea because these products were found to be tainted with benzo(a) pyrene, which is a known carcinogen.1 Several countries, including Philippines, Thailand, Taiwan and China, have been alerted to this and as a precaution, have removed these products from their shelves. Benzo(a)pyrene belongs to the group of polycyclic aromatic hydrocarbons (PAHs). Most PAHs derive from incomplete burning of carbon containing materials such as petroleum fuels, wood, and garbage. The occurrence of PAHs in foods is mainly caused by environmental contamination (water, air and soil), food processing (direct drying and heating), and packaging materials. PAHs have been found in different food products, such as dairy products, vegetables, fruits, oil, coffee, tea, cereals, smoked meat, seafood products, ingredients for food formulation, seasoning, and instant noodles. announcement of the test results and the recall order because the levels of In June, the KFDA conducted tests on benzopyrene found in the noodle products in June were minuscule and not harmful, 30 instant noodle products sold in the South Korean agency reported. However, the KFDA issued the recall order after South Korea and found that six its commissioner Lee Hee-sung was pressed on the matter during a legislative products contained benzopyrene, with question-and-answer, the report indicated.2 one product containing the highest level of 4.7 parts per billion (µg/kg). -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Polycyclic Aromatic Hydrocarbon Structure Index
NIST Special Publication 922 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899-0001 December 1997 revised August 2020 U.S. Department of Commerce William M. Daley, Secretary Technology Administration Gary R. Bachula, Acting Under Secretary for Technology National Institute of Standards and Technology Raymond G. Kammer, Director Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 This tabulation is presented as an aid in the identification of the chemical structures of polycyclic aromatic hydrocarbons (PAHs). The Structure Index consists of two parts: (1) a cross index of named PAHs listed in alphabetical order, and (2) chemical structures including ring numbering, name(s), Chemical Abstract Service (CAS) Registry numbers, chemical formulas, molecular weights, and length-to-breadth ratios (L/B) and shape descriptors of PAHs listed in order of increasing molecular weight. Where possible, synonyms (including those employing alternate and/or obsolete naming conventions) have been included. Synonyms used in the Structure Index were compiled from a variety of sources including “Polynuclear Aromatic Hydrocarbons Nomenclature Guide,” by Loening, et al. [1], “Analytical Chemistry of Polycyclic Aromatic Compounds,” by Lee et al. [2], “Calculated Molecular Properties of Polycyclic Aromatic Hydrocarbons,” by Hites and Simonsick [3], “Handbook of Polycyclic Hydrocarbons,” by J. R. Dias [4], “The Ring Index,” by Patterson and Capell [5], “CAS 12th Collective Index,” [6] and “Aldrich Structure Index” [7]. In this publication the IUPAC preferred name is shown in large or bold type. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Analysis of Benzopyrenes and Benzopyrene Metabolites by Fluorescence Spectroscopy Techniques
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2016 Analysis of Benzopyrenes and Benzopyrene Metabolites by Fluorescence Spectroscopy Techniques Bassam Al-Farhani University of Central Florida Part of the Chemistry Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Al-Farhani, Bassam, "Analysis of Benzopyrenes and Benzopyrene Metabolites by Fluorescence Spectroscopy Techniques" (2016). Electronic Theses and Dissertations, 2004-2019. 5286. https://stars.library.ucf.edu/etd/5286 ANALYSIS OF BENZOPYRENES AND BENZOPYRENE METABOLITES BY FLUORESCENCE SPECTROSCOPY TECHNIQUES by BASSAM AL-FARHANI B.Sc. Alnahrain University Iraq, 2002 M.Sc. Alnahrain University Iraq, 2005 A dissertation submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry in the College of Sciences at the University of Central Florida Orlando, Florida Spring Term 2016 Major Professor: Andres D. Campiglia © 2016 Bassam Al-farhani ii ABSTRACT Polycyclic aromatic hydrocarbons (PAHs) are some of the most common and toxic pollutants encountered worldwide. Presently, monitoring is restricted to sixteen PAHs, but it is well understood that this list omits many toxic PAHs. Among the “forgotten” PAHs, isomers with molecular weight 302 are of particular concern due to their high toxicological properties. The chromatographic analysis of PAHs with MW 302 is challenged by similar retention times and virtually identical mass fragmentation patterns. -

H2CS) and Its Thiohydroxycarbene Isomer (HCSH
A chemical dynamics study on the gas phase formation of thioformaldehyde (H2CS) and its thiohydroxycarbene isomer (HCSH) Srinivas Doddipatlaa, Chao Hea, Ralf I. Kaisera,1, Yuheng Luoa, Rui Suna,1, Galiya R. Galimovab, Alexander M. Mebelb,1, and Tom J. Millarc,1 aDepartment of Chemistry, University of Hawai’iatManoa, Honolulu, HI 96822; bDepartment of Chemistry and Biochemistry, Florida International University, Miami, FL 33199; and cSchool of Mathematics and Physics, Queen’s University Belfast, Belfast BT7 1NN, Northern Ireland, United Kingdom Edited by Stephen J. Benkovic, The Pennsylvania State University, University Park, PA, and approved August 4, 2020 (received for review March 13, 2020) Complex organosulfur molecules are ubiquitous in interstellar molecular sulfur dioxide (SO2) (21) and sulfur (S8) (22). The second phase clouds, but their fundamental formation mechanisms have remained commences with the formation of the central protostars. Tempera- largely elusive. These processes are of critical importance in initiating a tures increase up to 300 K, and sublimation of the (sulfur-bearing) series of elementary chemical reactions, leading eventually to organo- molecules from the grains takes over (20). The subsequent gas-phase sulfur molecules—among them potential precursors to iron-sulfide chemistry exploits complex reaction networks of ion–molecule and grains and to astrobiologically important molecules, such as the amino neutral–neutral reactions (17) with models postulating that the very acid cysteine. Here, we reveal through laboratory experiments, first sulfur–carbon bonds are formed via reactions involving methyl electronic-structure theory, quasi-classical trajectory studies, and astro- radicals (CH3)andcarbene(CH2) with atomic sulfur (S) leading to chemical modeling that the organosulfur chemistry can be initiated in carbonyl monosulfide and thioformaldehyde, respectively (18). -

The Life and Times of Carbon, Student Workbook
ELL Chemistry Assignment # 2 Name: __________________________________ Period: Teacher:_____________________ Collecting Carbon Lesson 1 | page 1 of 4 Where Can We Find Carbon? Carbon compounds are take up carbon dioxide from three-fourths of the dry weight everywhere around us. They the air, and use photosynthesis of plants is carbohydrates. In are in our foods, plastics, soils, to turn carbon dioxide into other words, when you look at in water, in the air, and in our carbohydrates. Carbohydrates plant matter (the stem, leaves, bodies. Carbon compounds can are carbon compounds that roots, and grains) you are come in three physical forms: store energy in the forms we call looking largely at carbohydrates. gas, liquid, and solid. Plants “starches” and “sugars.” About Animals (including humans) get their carbohydrates by eating plants, but they do not store much of what they consume. In fact, less than 1% of the body weight of animals is made up of carbohydrates. The main forms of carbon are: nonliving (abiotic) in rocks, soils, and sediments, and in water, such as bicarbonate, carbonate; living (biotic), such as plant and animal matter and dead organic matter; and carbon-based gases, such as carbon dioxide (CO2), methane (CH4), and carbon monoxide (CO). Chemists use a special system to describe compounds. They use a chemical formula of symbols that indicate the elements that make up chemical compounds. A chemical formula is also called a “molecular formula.” The chemical formula of carbon dioxide is CO2 indicating one carbon atom bonded to two oxygen atoms. The symbol for carbon is C; for hydrogen is H; for oxygen is O; for silica is Si. -

Table 2. Chemical Names and Alternatives, Abbreviations, and Chemical Abstracts Service Registry Numbers
Table 2. Chemical names and alternatives, abbreviations, and Chemical Abstracts Service registry numbers. [Final list compiled according to the National Institute of Standards and Technology (NIST) Web site (http://webbook.nist.gov/chemistry/); NIST Standard Reference Database No. 69, June 2005 release, last accessed May 9, 2008. CAS, Chemical Abstracts Service. This report contains CAS Registry Numbers®, which is a Registered Trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM] Aliphatic hydrocarbons CAS registry number Some alternative names n-decane 124-18-5 n-undecane 1120-21-4 n-dodecane 112-40-3 n-tridecane 629-50-5 n-tetradecane 629-59-4 n-pentadecane 629-62-9 n-hexadecane 544-76-3 n-heptadecane 629-78-7 pristane 1921-70-6 n-octadecane 593-45-3 phytane 638-36-8 n-nonadecane 629-92-5 n-eicosane 112-95-8 n-Icosane n-heneicosane 629-94-7 n-Henicosane n-docosane 629-97-0 n-tricosane 638-67-5 n-tetracosane 643-31-1 n-pentacosane 629-99-2 n-hexacosane 630-01-3 n-heptacosane 593-49-7 n-octacosane 630-02-4 n-nonacosane 630-03-5 n-triacontane 638-68-6 n-hentriacontane 630-04-6 n-dotriacontane 544-85-4 n-tritriacontane 630-05-7 n-tetratriacontane 14167-59-0 Table 2. Chemical names and alternatives, abbreviations, and Chemical Abstracts Service registry numbers.—Continued [Final list compiled according to the National Institute of Standards and Technology (NIST) Web site (http://webbook.nist.gov/chemistry/); NIST Standard Reference Database No. -
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom.