Experimental Evidence for Glycolaldehyde and Ethylene Glycol Formation by Surface Hydrogenation of CO Molecules Under Dense Molecular Cloud Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modelling Panspermia in the TRAPPIST-1 System
October 13, 2017 Modelling panspermia in the TRAPPIST-1 system James A. Blake1,2*, David J. Armstrong1,2, Dimitri Veras1,2 Abstract The recent ground-breaking discovery of seven temperate planets within the TRAPPIST-1 system has been hailed as a milestone in the development of exoplanetary science. Centred on an ultra-cool dwarf star, the planets all orbit within a sixth of the distance from Mercury to the Sun. This remarkably compact nature makes the system an ideal testbed for the modelling of rapid lithopanspermia, the idea that micro-organisms can be distributed throughout the Universe via fragments of rock ejected during a meteoric impact event. We perform N-body simulations to investigate the timescale and success-rate of lithopanspermia within TRAPPIST-1. In each simulation, test particles are ejected from one of the three planets thought to lie within the so-called ‘habitable zone’ of the star into a range of allowed orbits, constrained by the ejection velocity and coplanarity of the case in question. The irradiance received by the test particles is tracked throughout the simulation, allowing the overall radiant exposure to be calculated for each one at the close of its journey. A simultaneous in-depth review of space microbiological literature has enabled inferences to be made regarding the potential survivability of lithopanspermia in compact exoplanetary systems. 1Department of Physics, University of Warwick, Coventry, CV4 7AL 2Centre for Exoplanets and Habitability, University of Warwick, Coventry, CV4 7AL *Corresponding author: [email protected] Contents Universe, and can propagate from one location to another. This interpretation owes itself predominantly to the works of William 1 Introduction1 Thompson (Lord Kelvin) and Hermann von Helmholtz in the 1.1 Mechanisms for panspermia...............2 latter half of the 19th Century. -

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -
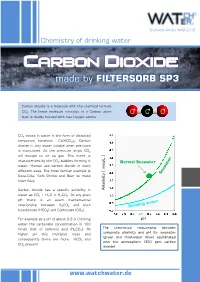
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Chemical Evolution Theory of Life's Origins the Lattimer, AST 248, Lecture 13 – P.2/20 Organics
Chemical Evolution Theory of Life's Origins 1. the synthesis and accumulation of small organic molecules, or monomers, such as amino acids and nucleotides. • Production of glycine (an amino acid) energy 3HCN+2H2O −→ C2H5O2N+CN2H2. • Production of adenine (a base): 5 HCN → C5H5N5, • Production of ribose (a sugar): 5H2CO → C5H10O5. 2. the joining of these monomers into polymers, including proteins and nucleic acids. Bernal showed that clay-like materials could serve as sites for polymerization. 3. the concentration of these molecules into droplets, called protobionts, that had chemical characteristics different from their surroundings. This relies heavily on the formation of a semi-permeable membrane, one that allows only certain materials to flow one way or the other through it. Droplet formation requires a liquid with a large surface tension, such as water. Membrane formation naturally occurs if phospholipids are present. 4. The origin of heredity, or a means of relatively error-free reproduction. It is widely, but not universally, believed that RNA-like molecules were the first self-replicators — the RNA world hypothesis. They may have been preceded by inorganic self-replicators. Lattimer, AST 248, Lecture 13 – p.1/20 Acquisition of Organic Material and Water • In the standard model of the formatio of the solar system, volatile materials are concentrated in the outer solar system. Although there is as much carbon as nearly all other heavy elements combined in the Sun and the bulk of the solar nebula, the high temperatures in the inner solar system have lead to fractional amounts of C of 10−3 of the average. -

Nomination Background: Dihydroxyacetone (CASRN: 96-26-4)
SUMMARY OF DATA FOR CHEMICAL SELECTION Dihydroxyacetone 96-26-4 BASIS OF NOMINATION TO THE CSWG As consumers have become more mindful of the hazards ofa "healthy tan," more individuals have turned to sunless tanning. Sunless tanning products represent about 10% of the $400 million market for suntan preparations, and these products are the fastest growing segment of the suntanning preparation market. All sunless tanners contain dihydroxyacetone. Information on the toxicity of dihydroxyacetone appears contradictory. A mutagen that induces DNA strand breaks, dihydroxyacetone is also an intermediate in carbohydrate metabolism in higher plants and animals. Such contradictions are not unprecedented, and it has been suggested that autooxidation of cx-hydroxycarbonyl compounds including reducing sugars may play a role in diseases associated with age and diabetes (Morita, 1991 ). When dihydroxyacetone was applied to the skin of mice, no carcinogenic effect was observed. It is unclear whether this negative response was caused by a failure ofthe compound to penetrate the skin. If so, extrapolating the dermal results to other routes of exposure would not be appropriate. NCI is nominating dihydroxyacetone to the NTP for dermal penetration studies in rats and mice to determine whether dihydroxyacetone can penetrate the skin. This information will clarify whether additional testing of dihydroxyacetone is warranted. Dihydroxyacetone 96-26-4 CHEMICAL IDENTIFICATION CAS Registry Number: 96-26-4 Chemical Abstracts Service Name: 1,3-Dihydroxy-2-propanone (9CI; 8CI) Synonyms and Tradenames: 1,3-Dihydroxydimethyl ketone; Chromelin; CTF A 00816; Dihyxal; Otan; Oxantin; Oxatone; Soleal; Triulose; Viticolor Structural Class: Ketone, ketotriose compound Structure. Molecular Formula. and Molecular Weight: 0 II /c"-. -

ETHYLENE GLYCOL: Environmental Aspects
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Concise International Chemical Assessment Document 22 ETHYLENE GLYCOL: Environmental aspects First draft prepared by Dr S. Dobson, Institute of Terrestrial Ecology, Natural Environment Research Council, Huntingdon, United Kingdom Please note that the layout and pagination of this pdf file are not identical to those of the printed CICAD Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2000 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organisation (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. -

Ethylene Glycol Ingestion Reviewer: Adam Pomerlau, MD Authors: Jeff Holmes, MD / Tammi Schaeffer, DO
Pediatric Ethylene Glycol Ingestion Reviewer: Adam Pomerlau, MD Authors: Jeff Holmes, MD / Tammi Schaeffer, DO Target Audience: Emergency Medicine Residents, Medical Students Primary Learning Objectives: 1. Recognize signs and symptoms of ethylene glycol toxicity 2. Order appropriate laboratory and radiology studies in ethylene glycol toxicity 3. Recognize and interpret blood gas, anion gap, and osmolal gap in setting of TA ingestion 4. Differentiate the symptoms and signs of ethylene glycol toxicity from those associated with other toxic alcohols e.g. ethanol, methanol, and isopropyl alcohol Secondary Learning Objectives: detailed technical/behavioral goals, didactic points 1. Perform a mental status evaluation of the altered patient 2. Formulate independent differential diagnosis in setting of leading information from RN 3. Describe the role of bicarbonate for severe acidosis Critical actions checklist: 1. Obtain appropriate diagnostics 2. Protect the patient’s airway 3. Start intravenous fluid resuscitation 4. Initiate serum alkalinization 5. Initiate alcohol dehydrogenase blockade 6. Consult Poison Center/Toxicology 7. Get Nephrology Consultation for hemodialysis Environment: 1. Room Set Up – ED acute care area a. Manikin Set Up – Mid or high fidelity simulator, simulated sweat if available b. Airway equipment, Sodium Bicarbonate, Nasogastric tube, Activated charcoal, IV fluid, norepinephrine, Simulated naloxone, Simulate RSI medications (etomidate, succinylcholine) 2. Distractors – ED noise For Examiner Only CASE SUMMARY SYNOPSIS OF HISTORY/ Scenario Background The setting is an urban emergency department. This is the case of a 2.5-year-old male toddler who presents to the ED with an accidental ingestion of ethylene glycol. The child was home as the father was watching him. The father was changing the oil on his car. -

H2CS) and Its Thiohydroxycarbene Isomer (HCSH
A chemical dynamics study on the gas phase formation of thioformaldehyde (H2CS) and its thiohydroxycarbene isomer (HCSH) Srinivas Doddipatlaa, Chao Hea, Ralf I. Kaisera,1, Yuheng Luoa, Rui Suna,1, Galiya R. Galimovab, Alexander M. Mebelb,1, and Tom J. Millarc,1 aDepartment of Chemistry, University of Hawai’iatManoa, Honolulu, HI 96822; bDepartment of Chemistry and Biochemistry, Florida International University, Miami, FL 33199; and cSchool of Mathematics and Physics, Queen’s University Belfast, Belfast BT7 1NN, Northern Ireland, United Kingdom Edited by Stephen J. Benkovic, The Pennsylvania State University, University Park, PA, and approved August 4, 2020 (received for review March 13, 2020) Complex organosulfur molecules are ubiquitous in interstellar molecular sulfur dioxide (SO2) (21) and sulfur (S8) (22). The second phase clouds, but their fundamental formation mechanisms have remained commences with the formation of the central protostars. Tempera- largely elusive. These processes are of critical importance in initiating a tures increase up to 300 K, and sublimation of the (sulfur-bearing) series of elementary chemical reactions, leading eventually to organo- molecules from the grains takes over (20). The subsequent gas-phase sulfur molecules—among them potential precursors to iron-sulfide chemistry exploits complex reaction networks of ion–molecule and grains and to astrobiologically important molecules, such as the amino neutral–neutral reactions (17) with models postulating that the very acid cysteine. Here, we reveal through laboratory experiments, first sulfur–carbon bonds are formed via reactions involving methyl electronic-structure theory, quasi-classical trajectory studies, and astro- radicals (CH3)andcarbene(CH2) with atomic sulfur (S) leading to chemical modeling that the organosulfur chemistry can be initiated in carbonyl monosulfide and thioformaldehyde, respectively (18). -

Ambient Water Quality Criteria for Polycyclic Aromatic Hydrocarbons (Pahs)
Ambient Water Quality Criteria For Polycyclic Aromatic Hydrocarbons (PAHs) Ministry of Environment, Lands and Parks Province of British Columbia N. K. Nagpal, Ph.D. Water Quality Branch Water Management Division February, 1993 ACKNOWLEDGEMENTS The author is indebted to the following individual and agencies for providing valuable comments during the preparation of this document. Dr. Ray Copes BC. Ministry of Health, Victoria, BC. Dr. G. R. Fox Environmental Protection Div., BC. MOELP, Victoria, BC. Mr. L. W. Pommen Water Quality Branch, BC. MOELP, Victoria, BC. Mr. R. J. Rocchini Water Quality Branch, BC. MOELP, Victoria, BC. Ms. Sherry Smith Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Mr. Scott Teed Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Ms. Bev Raymond Integrated Programs Branch, Inland Waters, Environment Canada, North Vancouver, BC. 1.0 INTRODUCTION Polycyclic aromatic hydrocarbons (PAHs) are organic compounds which are non- essential for the growth of plants, animals or humans; yet, they are ubiquitous in the environment. When present in sufficient quantity in the environment, certain PAHs are toxic and carcinogenic to plants, animals and humans. This document discusses the characteristics of PAHs and their effects on various water uses, which include drinking Ministry of Environment Water Protection and Sustainability Branch Mailing Address: Telephone: 250 387-9481 Environmental Sustainability PO Box 9362 Facsimile: 250 356-1202 and Strategic Policy Division Stn Prov Govt Website: www.gov.bc.ca/water Victoria BC V8W 9M2 water, aquatic life, wildlife, livestock watering, irrigation, recreation and aesthetics, and industrial water supplies. A significant portion of this document discusses the effects of PAHs upon aquatic life, due to its sensitivity to PAHs.