H2CS) and Its Thiohydroxycarbene Isomer (HCSH
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comprehensive Investigation on the Formation of Organo-Sulfur Molecules in Dark Clouds Via Neutral-Neutral Reactions
A&A 395, 1031–1044 (2002) Astronomy DOI: 10.1051/0004-6361:20021328 & c ESO 2002 Astrophysics A comprehensive investigation on the formation of organo-sulfur molecules in dark clouds via neutral-neutral reactions M. Yamada1, Y. Osamura1, and R. I. Kaiser2;? 1 Department of Chemistry, Rikkyo University, 3-34-1 Nishi-ikebukuro, Tokyo 171-8501, Japan 2 Department of Chemistry, University of York, YO10 5DD, UK Received 11 June 2002 / Accepted 9 September 2002 Abstract. Fifteen reactions on the doublet HCnS and singlet/triplet H2CnS(n = 1; 2) potential energy surfaces have been investi- gated theoretically via electronic structure calculations to unravel the formation of hydrogen deficient, sulfur bearing molecules via bimolecular reactions of two neutral species in cold molecular clouds. Various barrierless reaction pathways to synthe- size astronomically observed CnS and hitherto undetected HCnS(n = 1; 2) isomers are offered. These data present important 2 2 guidance to search for rotational transitions of the elusive thioformyl HCS(X A0) and thioketenyl HCCS(X Π) radicals in the interstellar medium. Key words. astrochemistry – ISM: molecules – molecular processes – methods: miscellaneous 1. Introduction terminated counterparts HCnSandCnSH have not been de- tected in the interstellar medium yet. However, these molecules Untangling the synthetic routes to form sulfur-carrying might represent the missing source of molecular-bound sul- molecules in the interstellar medium is a significant instru- fur in the interstellar medium. Hydrogen deficient organosul- ment to understand the chemical evolution and physical con- 2 2 fur molecules like HCS(X A0) and HCCS(X Π) resemble also ditions in star forming regions and in circumstellar envelopes important reaction intermediates which could lead to sulfur (Minh & van Dishoeck 2000). -
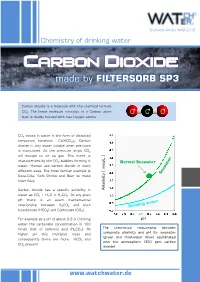
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Hydrogen Sulfide Public Health Statement
PUBLIC HEALTH STATEMENT Hydrogen Sulfide Division of Toxicology and Human Health Sciences December 2016 This Public Health Statement summarizes what is known about hydrogen sulfide such as possible health effects from exposure and what you can do to limit exposure. The U.S. Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites make up the National Priorities List (NPL) and are sites targeted for long-term federal clean-up activities. U.S. EPA has found hydrogen sulfide in at least 34 of the 1,832 current or former NPL sites. The total number of NPL sites evaluated for hydrogen sulfide is not known. But the possibility remains that as more sites are evaluated, the sites at which hydrogen sulfide is found may increase. This information is important because these future sites may be sources of exposure, and exposure to hydrogen sulfide may be harmful. If you are exposed to hydrogen sulfide, many factors determine whether you’ll be harmed. These include how much you are exposed to (dose), how long you are exposed (duration), and how you are exposed (route of exposure). You must also consider the other chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and state of health. WHAT IS HYDROGEN SULFIDE? Hydrogen sulfide (H2S) is a flammable, colorless gas that smells like rotten eggs. People usually can smell hydrogen sulfide at low concentrations in air, ranging from 0.0005 to 0.3 parts hydrogen sulfide per million parts of air (ppm). At high concentrations, a person might lose their ability to smell it. -

Ambient Water Quality Criteria for Polycyclic Aromatic Hydrocarbons (Pahs)
Ambient Water Quality Criteria For Polycyclic Aromatic Hydrocarbons (PAHs) Ministry of Environment, Lands and Parks Province of British Columbia N. K. Nagpal, Ph.D. Water Quality Branch Water Management Division February, 1993 ACKNOWLEDGEMENTS The author is indebted to the following individual and agencies for providing valuable comments during the preparation of this document. Dr. Ray Copes BC. Ministry of Health, Victoria, BC. Dr. G. R. Fox Environmental Protection Div., BC. MOELP, Victoria, BC. Mr. L. W. Pommen Water Quality Branch, BC. MOELP, Victoria, BC. Mr. R. J. Rocchini Water Quality Branch, BC. MOELP, Victoria, BC. Ms. Sherry Smith Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Mr. Scott Teed Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Ms. Bev Raymond Integrated Programs Branch, Inland Waters, Environment Canada, North Vancouver, BC. 1.0 INTRODUCTION Polycyclic aromatic hydrocarbons (PAHs) are organic compounds which are non- essential for the growth of plants, animals or humans; yet, they are ubiquitous in the environment. When present in sufficient quantity in the environment, certain PAHs are toxic and carcinogenic to plants, animals and humans. This document discusses the characteristics of PAHs and their effects on various water uses, which include drinking Ministry of Environment Water Protection and Sustainability Branch Mailing Address: Telephone: 250 387-9481 Environmental Sustainability PO Box 9362 Facsimile: 250 356-1202 and Strategic Policy Division Stn Prov Govt Website: www.gov.bc.ca/water Victoria BC V8W 9M2 water, aquatic life, wildlife, livestock watering, irrigation, recreation and aesthetics, and industrial water supplies. A significant portion of this document discusses the effects of PAHs upon aquatic life, due to its sensitivity to PAHs. -

UV-Spectrum of Thioformaldehyde-S-Oxide
Electronic Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2013 Matrix Isolation and Spectroscopic Properties • of the Methylsulfinyl Radical CH3(O)S [a] [b] [b] Hans Peter Reisenauer,* Jaroslaw Romanski, Grzegorz Mloston,* Peter R. Schreiner[a] [a] Justus-Liebig University, Institute of Organic Chemistry, Heinrich-Buff-Ring 58, D-35392 Giessen, Fax: (+49) 641-99-34209; E-mail: [email protected] [b] University of Lodz, Section of Heteroorganic Compounds, Tamka 12, PL-91-403 Lodz, Fax: (+48) (42) 665 51 62; E-mail: [email protected] Supplementary Information Table S1. Experimental (Ar, 10 K) and computed (AE-CCSD(T)/cc-pVTZ, harmonic approximation, no scaling) the IR spectra of 3. 13 3 C-3 D3-3 a b a b a b Sym. Approx. Band Position (Intensity) Band Position (Intensity) Band Position (Intensity) Mode Computation Experiment Computation Experiment Computation Experiment a’’ CH str. 3151.3 (2.1) 2995.4 3139.3 (2.1) n.o. 2336.4 (1.2) 2247.2 a’ CH str. 3149.3 (4.1) (vw) 3137.5 (4.0) n.o. 2334.5 (2.2) (vw) 2919.4 2095.5 a’ CH str. 3065.6 (4.3) 3062.7 (4.5) n.o. 2194.2 (1.4) (vw) (vw) CH 1062.8 a’ 3 1477.4 (6.5) 1417.1 (w) 1474.8 (6.6) 1414.6 (w) 1028.7 (s) def. (17.3) CH a’’ 3 1464.4 (7.8) 1405.2 (w) 1461.7 (7.8) 1401.4 (w) 1058.5 (3.7) 1025.6 (w) def. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Light Cycle Oil Other means of identification SDS number 106-GHS Synonyms Middle Distillate See section 16 for complete information. Recommended use Refinery feedstock. Recommended restrictions None known. Manufacturer/Importer/Supplier/Distributor information Manufacturer/Supplier Valero Marketing & Supply Company and Affiliates One Valero Way San Antonio, TX 78269-6000 General Assistance 210-345-4593 E-Mail [email protected] Contact Person Industrial Hygienist Emergency Telephone 24 Hour Emergency 866-565-5220 1-800-424-9300 (CHEMTREC USA) 2. Hazard(s) identification Physical hazards Flammable liquids Category 3 Health hazards Acute toxicity, inhalation Category 4 Skin corrosion/irritation Category 2 Carcinogenicity Category 1B Specific target organ toxicity, repeated Category 2 exposure Aspiration hazard Category 1 Environmental hazards Hazardous to the aquatic environment, Category 1 long-term hazard OSHA defined hazards Not classified. Label elements Signal word Danger Hazard statement Flammable liquid and vapor. Harmful if inhaled. Causes skin irritation. May cause cancer. May cause damage to organs (Blood, Liver, Kidney) through prolonged or repeated exposure. May be fatal if swallowed and enters airways. Precautionary statement Prevention Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Keep container tightly closed. Ground/bond container and receiving equipment. Use only non-sparking tools. Take precautionary measures against static discharges. Wear protective gloves/protective clothing/eye protection/face protection. Avoid breathing dust/fume/gas/mist/vapors/spray. Use only outdoors or in a well-ventilated area. Wash thoroughly after handling. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. -

Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)
City University of New York (CUNY) CUNY Academic Works Publications and Research City College of New York 2020 Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)-Releasing Synthetic Compounds as Novel Priming Agents against Drought Stress in Medicago sativa Plants Chrystalla Antoniou Cyprus University of Technology Rafaella Xenofontos Cyprus University of Technology Giannis Chatzimichail Cyprus University of Technology Anastasis Christou Agricultural Research Institute Khosrow Kashfi CUNY City College See next page for additional authors How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/cc_pubs/777 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Authors Chrystalla Antoniou, Rafaella Xenofontos, Giannis Chatzimichail, Anastasis Christou, Khosrow Kashfi, and Vasileios Fotopoulos This article is available at CUNY Academic Works: https://academicworks.cuny.edu/cc_pubs/777 biomolecules Article Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)-Releasing Synthetic Compounds as Novel Priming Agents against Drought Stress in Medicago sativa Plants Chrystalla Antoniou 1, Rafaella Xenofontos 1, Giannis Chatzimichail 1, Anastasis Christou 2, Khosrow Kashfi 3,4 and Vasileios Fotopoulos 1,* 1 Department of Agricultural Sciences, Biotechnology and Food Science, Cyprus University of Technology, 3603 Lemesos, Cyprus; [email protected] -

Hydrogen Sulfide Fact Sheet
Hydrogen Sulfide Fact Sheet What is hydrogen sulfide? Hydrogen sulfide (H 2S) occurs naturally in crude petroleum, natural gas, volcanic gases, and hot springs. It can also result from bacterial breakdown of organic matter. It is also produced by human and animal wastes. Bacteria found in your mouth and gastrointestinal tract produce hydrogen sulfide from bacteria decomposing materials that contain vegetable or animal proteins. Hydrogen sulfide can also result from industrial activities, such as food processing, coke ovens, kraft paper mills, tanneries, and petroleum refineries. Hydrogen sulfide is a flammable, colorless gas with a characteristic odor of rotten eggs. It is commonly known as hydrosulfuric acid, sewer gas, and stink damp. People can smell it at low levels. What happens to hydrogen sulfide when it enters the environment? • Hydrogen sulfide is released primarily as a gas and spreads in the air. • Hydrogen sulfide remains in the atmosphere for about 18 hours. • When released as a gas, it will change into sulfur dioxide and sulfuric acid. • In some instances, it may be released as a liquid waste from an industrial facility. How might I be exposed to hydrogen sulfide? • You may be exposed to hydrogen sulfide from breathing contaminated air or drinking contaminated water. • Individuals living near a wastewater treatment plant, a gas and oil drilling operation, a farm with manure storage or livestock confinement facilities, or a landfill may be exposed to higher levels of hydrogen sulfide. • You can be exposed at work if you work in the rayon textiles, petroleum and natural gas drilling and refining, or wastewater treatment industries. -

Hydrogen Sulfide and Nitric Oxide Are Mutually Dependent in The
Hydrogen sulfide and nitric oxide are mutually SEE COMMENTARY dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation Ciro Colettaa, Andreas Papapetropoulosa,b, Katalin Erdelyia, Gabor Olaha, Katalin Módisa, Panagiotis Panopoulosb, Antonia Asimakopouloub, Domokos Geröa, Iraida Sharinac, Emil Martinc, and Csaba Szaboa,1 aDepartment of Anesthesiology, University of Texas Medical Branch and Shriners Burns Hospital for Children, Galveston, TX 77555; bLaboratory of Molecular Pharmacology, Department of Pharmacy, University of Patras, 26504 Patras, Greece; and cDivision of Cardiology, Department of Internal Medicine, University of Texas Medical School at Houston, Houston, TX 77030 Edited by Solomon H. Snyder, The Johns Hopkins University School of Medicine, Baltimore, MD, and approved April 10, 2012 (received for review February 17, 2012) fi Hydrogen sulfide (H2S) is a unique gasotransmitter, with regula- of vascular tone and have signi cant implications for the patho- tory roles in the cardiovascular, nervous, and immune systems. genetic and therapeutic roles of both NO and H2S. Some of the vascular actions of H2S (stimulation of angiogenesis, relaxation of vascular smooth muscle) resemble those of nitric Results oxide (NO). Although it was generally assumed that H2S and NO H2S-Induced Angiogenesis Requires NO Biosynthesis. H2S resulted in exert their effects via separate pathways, the results of the current a concentration-dependent increase in bEnd3 microvascular en- A B study show that H2S and NO are mutually required to elicit angio- dothelial cell proliferation (Fig. S1 ) and migration (Fig. S1 and C), as well as microvessel sprouting from rat aortic rings (Fig. genesis and vasodilatation. Exposure of endothelial cells to H2S D increases intracellular cyclic guanosine 5′-monophosphate (cGMP) S1 ).