Common Equations Used in Chemistry Equation For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Configuring UNIX-Specific Settings: Creating Symbolic Links : Snap
Configuring UNIX-specific settings: Creating symbolic links Snap Creator Framework NetApp September 23, 2021 This PDF was generated from https://docs.netapp.com/us-en/snap-creator- framework/installation/task_creating_symbolic_links_for_domino_plug_in_on_linux_and_solaris_hosts.ht ml on September 23, 2021. Always check docs.netapp.com for the latest. Table of Contents Configuring UNIX-specific settings: Creating symbolic links . 1 Creating symbolic links for the Domino plug-in on Linux and Solaris hosts. 1 Creating symbolic links for the Domino plug-in on AIX hosts. 2 Configuring UNIX-specific settings: Creating symbolic links If you are going to install the Snap Creator Agent on a UNIX operating system (AIX, Linux, and Solaris), for the IBM Domino plug-in to work properly, three symbolic links (symlinks) must be created to link to Domino’s shared object files. Installation procedures vary slightly depending on the operating system. Refer to the appropriate procedure for your operating system. Domino does not support the HP-UX operating system. Creating symbolic links for the Domino plug-in on Linux and Solaris hosts You need to perform this procedure if you want to create symbolic links for the Domino plug-in on Linux and Solaris hosts. You should not copy and paste commands directly from this document; errors (such as incorrectly transferred characters caused by line breaks and hard returns) might result. Copy and paste the commands into a text editor, verify the commands, and then enter them in the CLI console. The paths provided in the following steps refer to the 32-bit systems; 64-bit systems must create simlinks to /usr/lib64 instead of /usr/lib. -

Humidity Definitions
ROTRONIC TECHNICAL NOTE Humidity Definitions 1 Relative humidity Table of Contents Relative humidity is the ratio of two pressures: %RH = 100 x p/ps where p is 1 Relative humidity the actual partial pressure of the water vapor present in the ambient and ps 2 Dew point / Frost the saturation pressure of water at the temperature of the ambient. point temperature Relative humidity sensors are usually calibrated at normal room temper - 3 Wet bulb ature (above freezing). Consequently, it generally accepted that this type of sensor indicates relative humidity with respect to water at all temperatures temperature (including below freezing). 4 Vapor concentration Ice produces a lower vapor pressure than liquid water. Therefore, when 5 Specific humidity ice is present, saturation occurs at a relative humidity of less than 100 %. 6 Enthalpy For instance, a humidity reading of 75 %RH at a temperature of -30°C corre - 7 Mixing ratio sponds to saturation above ice. by weight 2 Dew point / Frost point temperature The dew point temperature of moist air at the temperature T, pressure P b and mixing ratio r is the temperature to which air must be cooled in order to be saturated with respect to water (liquid). The frost point temperature of moist air at temperature T, pressure P b and mixing ratio r is the temperature to which air must be cooled in order to be saturated with respect to ice. Magnus Formula for dew point (over water): Td = (243.12 x ln (pw/611.2)) / (17.62 - ln (pw/611.2)) Frost point (over ice): Tf = (272.62 x ln (pi/611.2)) / (22.46 - -

Cygwin User's Guide
Cygwin User’s Guide Cygwin User’s Guide ii Copyright © Cygwin authors Permission is granted to make and distribute verbatim copies of this documentation provided the copyright notice and this per- mission notice are preserved on all copies. Permission is granted to copy and distribute modified versions of this documentation under the conditions for verbatim copying, provided that the entire resulting derived work is distributed under the terms of a permission notice identical to this one. Permission is granted to copy and distribute translations of this documentation into another language, under the above conditions for modified versions, except that this permission notice may be stated in a translation approved by the Free Software Foundation. Cygwin User’s Guide iii Contents 1 Cygwin Overview 1 1.1 What is it? . .1 1.2 Quick Start Guide for those more experienced with Windows . .1 1.3 Quick Start Guide for those more experienced with UNIX . .1 1.4 Are the Cygwin tools free software? . .2 1.5 A brief history of the Cygwin project . .2 1.6 Highlights of Cygwin Functionality . .3 1.6.1 Introduction . .3 1.6.2 Permissions and Security . .3 1.6.3 File Access . .3 1.6.4 Text Mode vs. Binary Mode . .4 1.6.5 ANSI C Library . .4 1.6.6 Process Creation . .5 1.6.6.1 Problems with process creation . .5 1.6.7 Signals . .6 1.6.8 Sockets . .6 1.6.9 Select . .7 1.7 What’s new and what changed in Cygwin . .7 1.7.1 What’s new and what changed in 3.2 . -

National Rappel Operations Guide
National Rappel Operations Guide 2019 NATIONAL RAPPEL OPERATIONS GUIDE USDA FOREST SERVICE National Rappel Operations Guide i Page Intentionally Left Blank National Rappel Operations Guide ii Table of Contents Table of Contents ..........................................................................................................................ii USDA Forest Service - National Rappel Operations Guide Approval .............................................. iv USDA Forest Service - National Rappel Operations Guide Overview ............................................... vi USDA Forest Service Helicopter Rappel Mission Statement ........................................................ viii NROG Revision Summary ............................................................................................................... x Introduction ...................................................................................................... 1—1 Administration .................................................................................................. 2—1 Rappel Position Standards ................................................................................. 2—6 Rappel and Cargo Letdown Equipment .............................................................. 4—1 Rappel and Cargo Letdown Operations .............................................................. 5—1 Rappel and Cargo Operations Emergency Procedures ........................................ 6—1 Documentation ................................................................................................ -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Μmachine Definitions
Machine/Code For the final part of the CS450 project, you are to complete a fully functional Pascal compiler. Since it would be impractical to have you generate assembly code for a real machine (with all the intricacies of the target machine), we have created a virtual machine that has been designed specifically for a Pascal compiler. The Machine (and is associated assembly language Code) greatly simplifies the task of code generation while still requiring you to handle many of the problems faced by other compiler writers. At this point in time, you should have written a scanner and parser for Pascal, should be working on the symbol table and should be thinking about semantic processing and code generation. The following information about the Machine and Code is provided to assist you in your design and implementation of the remaining parts of the Pascal compiler project: Machine Specification: The Machine is a virtual machine (simulated by a program) with the following hardware characteristics: Separate instruction space (for assembly code) and RAM (for data storage/retrieval) 10 general purpose registers (D0 - D9) Special stack pointer register (SP) The Machine is a stack-based machine; all memory is allocated/deallocated on the data stack residing in RAM: The data stack supports types: Integer, Float/Fixed, Strings. All data types have the same size of 1. The data stack grows upwards (starts at 0, pushes increment the SP, pops decrement the SP) Supported Data Types Integer: As defined in the Pascal tokens document. Size: 1. Float/Fixed: Numbers represented as floating point or fixed point are supported and have a size of 1. -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

Outbreak of SARS-Cov-2 Infections, Including COVID-19 Vaccine
Morbidity and Mortality Weekly Report Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings — Barnstable County, Massachusetts, July 2021 Catherine M. Brown, DVM1; Johanna Vostok, MPH1; Hillary Johnson, MHS1; Meagan Burns, MPH1; Radhika Gharpure, DVM2; Samira Sami, DrPH2; Rebecca T. Sabo, MPH2; Noemi Hall, PhD2; Anne Foreman, PhD2; Petra L. Schubert, MPH1; Glen R. Gallagher PhD1; Timelia Fink1; Lawrence C. Madoff, MD1; Stacey B. Gabriel, PhD3; Bronwyn MacInnis, PhD3; Daniel J. Park, PhD3; Katherine J. Siddle, PhD3; Vaira Harik, MS4; Deirdre Arvidson, MSN4; Taylor Brock-Fisher, MSc5; Molly Dunn, DVM5; Amanda Kearns5; A. Scott Laney, PhD2 On July 30, 2021, this report was posted as an MMWR Early Massachusetts, that attracted thousands of tourists from across Release on the MMWR website (https://www.cdc.gov/mmwr). the United States. Beginning July 10, the Massachusetts During July 2021, 469 cases of COVID-19 associated Department of Public Health (MA DPH) received reports of with multiple summer events and large public gatherings in an increase in COVID-19 cases among persons who reside in a town in Barnstable County, Massachusetts, were identified or recently visited Barnstable County, including in fully vac- among Massachusetts residents; vaccination coverage among cinated persons. Persons with COVID-19 reported attending eligible Massachusetts residents was 69%. Approximately densely packed indoor and outdoor events at venues that three quarters (346; 74%) of cases occurred in fully vac- included bars, restaurants, guest houses, and rental homes. On cinated persons (those who had completed a 2-dose course July 3, MA DPH had reported a 14-day average COVID-19 of mRNA vaccine [Pfizer-BioNTech or Moderna] or had incidence of zero cases per 100,000 persons per day in residents received a single dose of Janssen [Johnson & Johnson] vac- of the town in Barnstable County; by July 17, the 14-day cine ≥14 days before exposure). -
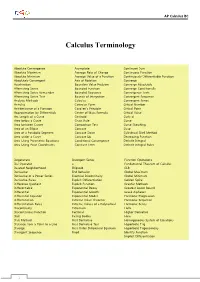
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Linux Cheat Sheet
1 of 4 ########################################### # 1.1. File Commands. # Name: Bash CheatSheet # # # # A little overlook of the Bash basics # ls # lists your files # # ls -l # lists your files in 'long format' # Usage: A Helpful Guide # ls -a # lists all files, including hidden files # # ln -s <filename> <link> # creates symbolic link to file # Author: J. Le Coupanec # touch <filename> # creates or updates your file # Date: 2014/11/04 # cat > <filename> # places standard input into file # Edited: 2015/8/18 – Michael Stobb # more <filename> # shows the first part of a file (q to quit) ########################################### head <filename> # outputs the first 10 lines of file tail <filename> # outputs the last 10 lines of file (-f too) # 0. Shortcuts. emacs <filename> # lets you create and edit a file mv <filename1> <filename2> # moves a file cp <filename1> <filename2> # copies a file CTRL+A # move to beginning of line rm <filename> # removes a file CTRL+B # moves backward one character diff <filename1> <filename2> # compares files, and shows where differ CTRL+C # halts the current command wc <filename> # tells you how many lines, words there are CTRL+D # deletes one character backward or logs out of current session chmod -options <filename> # lets you change the permissions on files CTRL+E # moves to end of line gzip <filename> # compresses files CTRL+F # moves forward one character gunzip <filename> # uncompresses files compressed by gzip CTRL+G # aborts the current editing command and ring the terminal bell gzcat <filename> # -

Alphabets, Letters and Diacritics in European Languages (As They Appear in Geography)
1 Vigleik Leira (Norway): [email protected] Alphabets, Letters and Diacritics in European Languages (as they appear in Geography) To the best of my knowledge English seems to be the only language which makes use of a "clean" Latin alphabet, i.d. there is no use of diacritics or special letters of any kind. All the other languages based on Latin letters employ, to a larger or lesser degree, some diacritics and/or some special letters. The survey below is purely literal. It has nothing to say on the pronunciation of the different letters. Information on the phonetic/phonemic values of the graphic entities must be sought elsewhere, in language specific descriptions. The 26 letters a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r, s, t, u, v, w, x, y, z may be considered the standard European alphabet. In this article the word diacritic is used with this meaning: any sign placed above, through or below a standard letter (among the 26 given above); disregarding the cases where the resulting letter (e.g. å in Norwegian) is considered an ordinary letter in the alphabet of the language where it is used. Albanian The alphabet (36 letters): a, b, c, ç, d, dh, e, ë, f, g, gj, h, i, j, k, l, ll, m, n, nj, o, p, q, r, rr, s, sh, t, th, u, v, x, xh, y, z, zh. Missing standard letter: w. Letters with diacritics: ç, ë. Sequences treated as one letter: dh, gj, ll, rr, sh, th, xh, zh. -
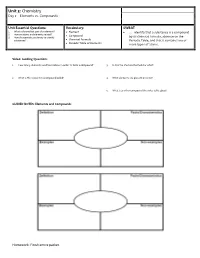
Unit 3: Chemistry Day 2 – Elements Vs
Unit 3: Chemistry Day 2 – Elements vs. Compounds Unit Essential Questions: Vocabulary: SWBAT 1. What is the smallest part of an element? Element … identify that a substance is a compound 2. How are atoms and elements related? Compound 3. How do scientists use density to identify by its chemical formula, absence on the substances? Chemical Formula Periodic Table, and that it contains two or Periodic Table of Elements more types of atoms. Video: Guiding Questions 1. How many elements need to combine in order to form a compound? 3. H2O is the chemical formula for what? 2. What is the recipe for a compound called? 4. What elements are present in water? 5. What is another compound the video talks about? GUIDED NOTES: Elements and Compounds Homework: Finish entire packet. STOP AND JOT: What is the difference between an element and a compound? Chemical Formulas Compounds are represented by _________________________________ _____________________________________. Each capital letter represents _______________ element. ____________________________________ represent the number of atoms of each element. Examples: H2O Si3N4 Number of elements: _____________________ Number of elements: _____________________ Number of atoms for each element: Number of atoms for each element: ______________________ ______________________ ______________________ ________________________ How many elements are in How many atoms of each How many elements are in How many atoms of each the compound H3PO4? element is present in the the compound C2H5OH? element is present in the compound, HNO3? compound, C2H4O2? Three ways to identify a Compound: 1. Made of more than one _________________________. 2. Not located on the _____________________________ __________________________ _________________ ________________________________. 3. Represented by a ____________________________________ ____________________________________.