Ethyl Alcohol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Solvents for Pahs Extraction and Enhancement of the Pahs Bioremediation in Coal- Tar-Contaminated Soils Pak-Hing Lee Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2000 Use of solvents for PAHs extraction and enhancement of the PAHs bioremediation in coal- tar-contaminated soils Pak-Hing Lee Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Environmental Engineering Commons Recommended Citation Lee, Pak-Hing, "Use of solvents for PAHs extraction and enhancement of the PAHs bioremediation in coal-tar-contaminated soils " (2000). Retrospective Theses and Dissertations. 13912. https://lib.dr.iastate.edu/rtd/13912 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter fece, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quaiity of the copy submitted. Broken or indistinct print colored or poor quality illustrations and photographs, print bleedthrough, substeindard margins, and improper alignment can adversely affect reproduction. In the unlilcely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Safer Cocktail for an Ethanol/Water/Ammonia Solvent
L A S C P LSC in Practice C P O C L Safer Cocktail for an Ethanol/Water/ K T I A I C Ammonia Solvent Mixture L S A Problem T A laboratory had been under pressure to move towards Using this mixture, the sample uptake capacity of I the newer generation of safer liquid scintillation ULTIMA-Flo AP, ULTIMA-Flo M and ULTIMA Gold O cocktails. Unfortunately, the sample composition of LLT (PerkinElmer 6013599, 6013579 and 6013377, one of their targets kept giving the researchers problems. respectively) at 20 °C was determined and N Before the lead researcher contacted PerkinElmer, the results were: they had tried PerkinElmer’s Opti-Fluor®, ULTIMA ULTIMA-Flo AP 4.00 mL in 10 mL cocktail N Gold LLT, and ULTIMA Gold XR. All of the safer O ™ ULTIMA-Flo M 4.25 mL in 10 mL cocktail cocktails could incorporate each of the constituents T as indicated by their sample capacity graphs. ULTIMA Gold LLT 4.25 mL in 10 mL cocktail E The problematic sample was an extraction solvent From these results, we observed that it was possible consisting of 900 mL ethanol, 50 mL ammonium to get 4.0 mL of the sample into all of these cocktails. hydroxide, 500 mL water and an enzyme containing In addition, we determined that it was not possible to 14C. The mixture had a pH in the range of 10 to 11. get 4 mL sample into 7 mL of any of these cocktails. To complete this work, we also checked for lumines- Discussion cence using 4 mL of sample in 10 mL of each cocktail. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Fuel Properties Comparison
Alternative Fuels Data Center Fuel Properties Comparison Compressed Liquefied Low Sulfur Gasoline/E10 Biodiesel Propane (LPG) Natural Gas Natural Gas Ethanol/E100 Methanol Hydrogen Electricity Diesel (CNG) (LNG) Chemical C4 to C12 and C8 to C25 Methyl esters of C3H8 (majority) CH4 (majority), CH4 same as CNG CH3CH2OH CH3OH H2 N/A Structure [1] Ethanol ≤ to C12 to C22 fatty acids and C4H10 C2H6 and inert with inert gasses 10% (minority) gases <0.5% (a) Fuel Material Crude Oil Crude Oil Fats and oils from A by-product of Underground Underground Corn, grains, or Natural gas, coal, Natural gas, Natural gas, coal, (feedstocks) sources such as petroleum reserves and reserves and agricultural waste or woody biomass methanol, and nuclear, wind, soybeans, waste refining or renewable renewable (cellulose) electrolysis of hydro, solar, and cooking oil, animal natural gas biogas biogas water small percentages fats, and rapeseed processing of geothermal and biomass Gasoline or 1 gal = 1.00 1 gal = 1.12 B100 1 gal = 0.74 GGE 1 lb. = 0.18 GGE 1 lb. = 0.19 GGE 1 gal = 0.67 GGE 1 gal = 0.50 GGE 1 lb. = 0.45 1 kWh = 0.030 Diesel Gallon GGE GGE 1 gal = 1.05 GGE 1 gal = 0.66 DGE 1 lb. = 0.16 DGE 1 lb. = 0.17 DGE 1 gal = 0.59 DGE 1 gal = 0.45 DGE GGE GGE Equivalent 1 gal = 0.88 1 gal = 1.00 1 gal = 0.93 DGE 1 lb. = 0.40 1 kWh = 0.027 (GGE or DGE) DGE DGE B20 DGE DGE 1 gal = 1.11 GGE 1 kg = 1 GGE 1 gal = 0.99 DGE 1 kg = 0.9 DGE Energy 1 gallon of 1 gallon of 1 gallon of B100 1 gallon of 5.66 lb., or 5.37 lb. -

Liquichek Ethanol/Ammonia Control SERUM CHEMISTRY CONTROLS Liquichek Ethanol/Ammonia Control
Bio-Rad Laboratories SERUM CHEMISTRY CONTROLS Liquichek Ethanol/Ammonia Control SERUM CHEMISTRY CONTROLS Liquichek Ethanol/Ammonia Control A liquid control used to monitor the precision of Ethanol and Ammonia test procedures in the clinical laboratory. • Liquid • Normal, elevated and toxic concentrations of Ammonia • 2 year shelf life at 2–8°C • 20 day open-vial stability at 2–8°C Analytes Ammonia Ethanol Refer to the package insert of currently available lots for specific analyte and stability claims Ordering Information Cat # Description 544 Level 1 ..................................................................................... 6 x 3 mL 545 Level 2 ..................................................................................... 6 x 3 mL 546 Level 3 ..................................................................................... 6 x 3 mL 545X Trilevel MiniPak (1 of each level) .................................................................. 3 x 3 mL EQAS Independent QC Unity An independent, external assessment of laboratory Ongoing, proactive, unbiased daily QC that QC Data Management tools that help you create performance in comparison to your peers. helps identify errors as they occur or begin to trend. a strategy to reduce risk and streamline QC workflow. QCNet.com/eqas QCNet.com/independentqc QCNet.com/datamanagement Bio-Rad For further information, please contact the Bio-Rad office nearest you Laboratories, Inc. or visit our website at www.bio-rad.com/qualitycontrol Clinical Website www.bio-rad.com/diagnostics Australia -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

Cellulosic Ethanol Production Via Aqueous Ammonia Soaking Pretreatment and Simultaneous Saccharification and Fermentation Asli Isci Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2008 Cellulosic ethanol production via aqueous ammonia soaking pretreatment and simultaneous saccharification and fermentation Asli Isci Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agriculture Commons, and the Bioresource and Agricultural Engineering Commons Recommended Citation Isci, Asli, "Cellulosic ethanol production via aqueous ammonia soaking pretreatment and simultaneous saccharification and fermentation" (2008). Retrospective Theses and Dissertations. 15695. https://lib.dr.iastate.edu/rtd/15695 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Cellulosic ethanol production via aqueous ammonia soaking pretreatment and simultaneous saccharification and fermentation by Asli Isci A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Co-majors: Agricultural and Biosystems Engineering; Biorenewable Resources and Technology Program of Study Committee: Robert P. Anex, Major Professor D. Raj Raman Anthony L. Pometto III. Kenneth J. Moore Robert C. Brown Iowa State University Ames, Iowa -
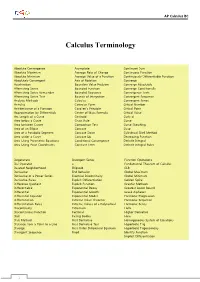
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Dehydrogenation of Ethanol to Acetaldehyde Over Different Metals Supported on Carbon Catalysts
catalysts Article Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts Jeerati Ob-eye , Piyasan Praserthdam and Bunjerd Jongsomjit * Center of Excellence on Catalysis and Catalytic Reaction Engineering, Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] (J.O.-e.); [email protected] (P.P.) * Correspondence: [email protected]; Tel.: +66-2-218-6874 Received: 29 November 2018; Accepted: 27 December 2018; Published: 9 January 2019 Abstract: Recently, the interest in ethanol production from renewable natural sources in Thailand has been receiving much attention as an alternative form of energy. The low-cost accessibility of ethanol has been seen as an interesting topic, leading to the extensive study of the formation of distinct chemicals, such as ethylene, diethyl ether, acetaldehyde, and ethyl acetate, starting from ethanol as a raw material. In this paper, ethanol dehydrogenation to acetaldehyde in a one-step reaction was investigated by using commercial activated carbon with four different metal-doped catalysts. The reaction was conducted in a packed-bed micro-tubular reactor under a temperature range of 250–400 ◦C. The best results were found by using the copper doped on an activated carbon catalyst. Under this specified condition, ethanol conversion of 65.3% with acetaldehyde selectivity of 96.3% at 350 ◦C was achieved. This was probably due to the optimal acidity of copper doped on the activated carbon catalyst, as proven by the temperature-programmed desorption of ammonia (NH3-TPD). In addition, the other three catalyst samples (activated carbon, ceria, and cobalt doped on activated carbon) also favored high selectivity to acetaldehyde (>90%). -

Ethylene Glycol Ingestion Reviewer: Adam Pomerlau, MD Authors: Jeff Holmes, MD / Tammi Schaeffer, DO
Pediatric Ethylene Glycol Ingestion Reviewer: Adam Pomerlau, MD Authors: Jeff Holmes, MD / Tammi Schaeffer, DO Target Audience: Emergency Medicine Residents, Medical Students Primary Learning Objectives: 1. Recognize signs and symptoms of ethylene glycol toxicity 2. Order appropriate laboratory and radiology studies in ethylene glycol toxicity 3. Recognize and interpret blood gas, anion gap, and osmolal gap in setting of TA ingestion 4. Differentiate the symptoms and signs of ethylene glycol toxicity from those associated with other toxic alcohols e.g. ethanol, methanol, and isopropyl alcohol Secondary Learning Objectives: detailed technical/behavioral goals, didactic points 1. Perform a mental status evaluation of the altered patient 2. Formulate independent differential diagnosis in setting of leading information from RN 3. Describe the role of bicarbonate for severe acidosis Critical actions checklist: 1. Obtain appropriate diagnostics 2. Protect the patient’s airway 3. Start intravenous fluid resuscitation 4. Initiate serum alkalinization 5. Initiate alcohol dehydrogenase blockade 6. Consult Poison Center/Toxicology 7. Get Nephrology Consultation for hemodialysis Environment: 1. Room Set Up – ED acute care area a. Manikin Set Up – Mid or high fidelity simulator, simulated sweat if available b. Airway equipment, Sodium Bicarbonate, Nasogastric tube, Activated charcoal, IV fluid, norepinephrine, Simulated naloxone, Simulate RSI medications (etomidate, succinylcholine) 2. Distractors – ED noise For Examiner Only CASE SUMMARY SYNOPSIS OF HISTORY/ Scenario Background The setting is an urban emergency department. This is the case of a 2.5-year-old male toddler who presents to the ED with an accidental ingestion of ethylene glycol. The child was home as the father was watching him. The father was changing the oil on his car. -
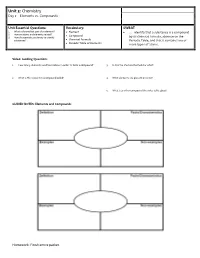
Unit 3: Chemistry Day 2 – Elements Vs
Unit 3: Chemistry Day 2 – Elements vs. Compounds Unit Essential Questions: Vocabulary: SWBAT 1. What is the smallest part of an element? Element … identify that a substance is a compound 2. How are atoms and elements related? Compound 3. How do scientists use density to identify by its chemical formula, absence on the substances? Chemical Formula Periodic Table, and that it contains two or Periodic Table of Elements more types of atoms. Video: Guiding Questions 1. How many elements need to combine in order to form a compound? 3. H2O is the chemical formula for what? 2. What is the recipe for a compound called? 4. What elements are present in water? 5. What is another compound the video talks about? GUIDED NOTES: Elements and Compounds Homework: Finish entire packet. STOP AND JOT: What is the difference between an element and a compound? Chemical Formulas Compounds are represented by _________________________________ _____________________________________. Each capital letter represents _______________ element. ____________________________________ represent the number of atoms of each element. Examples: H2O Si3N4 Number of elements: _____________________ Number of elements: _____________________ Number of atoms for each element: Number of atoms for each element: ______________________ ______________________ ______________________ ________________________ How many elements are in How many atoms of each How many elements are in How many atoms of each the compound H3PO4? element is present in the the compound C2H5OH? element is present in the compound, HNO3? compound, C2H4O2? Three ways to identify a Compound: 1. Made of more than one _________________________. 2. Not located on the _____________________________ __________________________ _________________ ________________________________. 3. Represented by a ____________________________________ ____________________________________. -

Ide and Ethanol Rocket Engine
INJECTOR AND COMBUSTION CHAMBER DESIGN FOR A NITROUS OX- IDE AND ETHANOL ROCKET ENGINE Giovani Hidalgo Ceotto Escola Politécnica - Universidade de São Paulo [email protected] Guilherme Castrignano Tavares Escola Politécnica - Universidade de São Paulo [email protected] Abstract. The basic design of a rocket engine injector and combustion chamber for saturated nitrous oxide and liquid ethanol is presented. At first, an oxidant-fuel mixture is selected based on a thermochemical analysis that explores several existing options and other combinations that have not yet been studied. As a result, nitrous oxide is chosen as an oxidant and ethanol as fuel. Then a simplified methodology is proposed for the design of a pressure-swirl injector responsible for ethanol. Computational fluid dynamics is used to verify the validity of the above-mentioned analysis, using Volume of Fluid (VOF). For the nitrous oxide injector, the flash-boiling phenomenon is investigated, verifying its importance for the ongoing project. The effect is treated analytically using the Dyer model to account for non-equilibrium thermodynamics. Simplified zero-dimensional and one-dimensional combustion models are explored as tools to design the rocket combustion chamber. Furthermore, combustion instability due to acoustic phenomena is studied, with the first spinning tangential mode being computed for the herein developed motor and an ensemble of acoustic cavities being developed to suppress the aforementioned mode. Finally, a diagram of the static test bench which will be used to validate the injectors and the designed engine is also presented. Keywords: Liquid rocket motor, Injector, Combustion, Instability, Acoustic cavity. 1. INTRODUCTION The aerospace sector has been growing in the past years with the introduction of new private-owned companies explor- ing this market that was previously dominated by governments.