ASPEN Safe Practices for Enteral Nutrition Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adult Tube Feeding
Guide to ADULT TUBE FEEDING Parents’ Practical Guide to Pediatric Tube Feeding | XX Contents Introduction 3 Finding Community Support 4 Understanding the Tube Feeding System 6 Monitoring Your Response to Tube Feeding 8 Taking Charge of Your Doctor Visits 18 Tube Feeding Monitoring Checklist 20 Medication Record 28 Notes 30 Glossary 32 Guide to Adult Tube Feeding | 1 Introduction We know that tube feeding brings major changes to your life. But you don’t have to face them alone. We hope you find this guide a useful, practical resource that can help you tube feed successfully at home. You’ll find step-by-step instructions on handling issues you face every day, from coping with infections to preparing for a doctor’s appointment. The guide includes worksheets (P. 20-31) that make it simple to record important information about your progress. We’ve also added a helpful glossary (P. 32-34) that you can refer to if you come across any unfamiliar terms. While technical and medical support form the foundation of tube-feeding success, we believe that emotional support is just as important. Hopefully, you’ll find resources in this guide that make your journey easier. Guide to Adult Tube Feeding | 3 Finding Community Support With support and guidance, you can take control of the tube-feeding process and adjust successfully to this new lifestyle change. Visit the link below to find educational resources, support groups and the opportunity to connect with others in your situation. The Oley Foundation The Oley Foundation is a nonprofit organization for people who depend on home enteral (tube) feeding or parenteral (intravenous) feeding. -

Nutrition Department This Booklet Has Been Developed by the Nutrition and Gastroenterology Department’S at Alfred Health, Melbourne
Nutrition Department This booklet has been developed by the Nutrition and Gastroenterology Department’s at Alfred Health, Melbourne. Inside you will find information on tube feeding at home CONTENTS 1. Your tube & feeding regime Tube details page 1 Feeding regime page 1 2. Important contact phone numbers page 1 3. Introduction What is tube feeding? page 2 Who receives tube feeding? page 2 4. The feeding tube Nasogastric tube page 3 Nasojejunal tube page 3 Gastrostomy tubes page 3—7 Jejunostomy tubes page 8 Trans-gastric jejunostomy tubes page 8 5. The formula Formula selection & feeding plan page 9 - 10 Formula storage & preparation page 10 6. Feeding methods Continuous OR Intermittent feeding using a pump page 11 - 12 Continuous OR Intermittent feeding using gravity drip page 13 - 14 Bolus feeding page 15 - 16 Oral feeding page 16 7. Medication Administration of medication page 17 8. Care during tube feeding Gastrostomy feeding tube care: Care immediately post tube insertion page 18 Daily tube & stoma care page 19 Jejunostomy, trans-gastric jejunostomy & PEG—J page 20 Nasogastric tube care page 20 Care of the tube feeding equipment page 21 Mouth care page 21 9. Possible problems & solutions Blocked Tube page 22 Constipation page 22 - 23 Diarrhoea page 23 - 24 Irritation, skin redness &/or oozing page 24 Leaking around tube page 24 Nausea & vomiting page 25 Reflux page 25 Tube dislodged or falls out page 25 Tube deteriorated or damaged page 25 What to do if your feeding tube has fallen out page 26 10. The Alfred Home Enteral Nutrition (HEN) program Requirements of the HEN Program page 27 The PEG/HEN Clinic page 28 Ordering formula & equipment pager 28 11. -

JEJUNOSTOMY Feeding Tube PASSPORT (JEJ)
Hull University Teaching Hospitals NHS Trust JEJUNOSTOMY Feeding Tube PASSPORT (JEJ) Tube INFORMATION ABOUT MY JEJUNOSTOMY FEEDING TUBE Affix Addressograph Has a tube How inserted? Site of bowel insertion: e.g. Jejunum, Terminal Ileum (Circle) Date inserted: Skin Suture Removal Date Yes / No Weekly Balloon change (If required) Abdominal measurement (If required) cm Type of feed: Continuous/mls per hour mls Flush with of sterile water pre & post feed & medication. 30mls Additional flushes can be given as indicated by your dietitian. Long term plan If during the first 7 days following your tube insertion, you notice any leak of fluid around the tube, pain on feeding, flushing or if there is fresh bleeding, STOP the feed immediately and contact Ward 14 Castle Hill Hospital - see contact numbers on page 16 2 CONTENTS Page Going home with a jejunostomy tube 4 What is a feeding jejunostomy tube 4 How long will I need it? 5 Surgically placed jejunostomy tube with stitches 5 & 6 Jejunostomy tube with balloon 6 General care / stoma care 7 Flushing 8 Pump feeding/Key Points 9 My Feed regime 10 & 11 Tube blockage 12 Tube fallen out 12 Mouth care 13 Medicine 13 Feed storage and disposal 13 Training prior to going home 14 Going home 14 Equipment for discharge 15 When discharged from hospital 15 Contact numbers between 9am-5pm 16 Emergency contact details after 5pm 16 This booklet contains useful information and advice for patients leaving hospital with a Jejunostomy feeding tube. How it works and how to maintain it. It also lists specific interventions of what to do should you encounter any problems. -

Medications That Are Safe During Pregnancy
Medications that Are Safe during Pregnancy Women who are between four and 12 weeks pregnant may safely take the following over-the-counter medications. Follow all directions on the container for adult dosage/use. ~r---------~ I Problem Over the Counter ICall Your Care Provider for: c __ ; Morning sickness Vitamin 86: take 50 mg/day to start; if not Persistent vomiting; weight loss or helpful, increase by 50 mg 2 to 4 times/day inability to tolerate fluids for 24 hours until you reach a total of 200 mg/day. Do not take more than 200 mg each day. I Increase fluids. i I Mild headaches / general Try comfort measures. Severe and/or persistent headaches I aches & pains Acetominophen (Tylenol) - ~ ~""~'" I Nasal congestion due to a IOcean Mist nasal spray I cold, sinusitis or allergies I I -, Women who are more than 12 weeks pregnant may safely take the following over-the-counter medications. Follow all directions on the container for adult dosage/use. I Problem lover the Counter 1Call Your Care Provider for: '" '''''Moo "0'''"0'''''''''''" , Nasal congestion due to a Sudafed, Afrin nasal spray, Ocean Mist cold, sinusitis or allergies nasal spray I I i Cough due to minor throat Robitussin (or other brand of Guaifenesin), Persistent cough i irritation Robitussin DM or non-alcohol cough syrup (not to exceed 1 ! week's use) i I Nasal congestion and coUgh Triaminic DM (or other brand of alcohol- free and antihistamine-free decongestant I and antitussive) I I I Sore throat Alcohol-free lozenges, such as Severe or persistent sore throat Chloraseptic -

Tube Feeding Protocol: Supporting an Individual with a Feeding Tube
Tube Feeding Protocol: Supporting an Individual with a Feeding Tube Introduction Some people may be unable to take foods or fluids by mouth due to dysphagia. Others may require supplementation because they are unable to take sufficient foods or fluids by mouth, and formula delivered through a feeding tube may provide them with much needed additional nutrients. It is helpful if guidelines (A Tube Feeding Protocol) are in place prior to the need for this intervention. Below are some suggested guidelines for supporting an Individual with a feeding tube. Information to be documented by the physician The reason (medical diagnosis) requiring feeding tube insertion Type of feeding tube inserted Types of feeding tubes The Nasogastric Tube (NG tube): Passed into either nostril, down the esophagus and into the stomach. This is used for short term feedings. The Gastrostomy tube (G - tube or PEG): Surgically placed through the abdominal wall into the stomach. The tube will be located below the rib cage and to the left. The Jejunostomy tube (J - tube or PEJ): Surgically implanted in the upper portion of the jejunum (Part of the small intestine.) The tube will be located lower in the abdomen and more toward the center than the G – tube. Feedings through a J – tube must always be by pump. The Gastrostomy-Jejunostomy (GJ - tube): Surgically placed in the stomach, like the G – tube, but the tubing is longer, the end is in the jejunum, and there are two ports. Feeding technique Feeding techniques Bolus: A set amount of formula is given over a short period of time via syringe. -
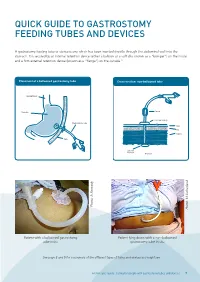
Quick Guide to Gastrostomy Feeding Tubes and Devices
QUICK GUIDE TO GASTROSTOMY FEEDING TUBES AND DEVICES A gastrostomy feeding tube or device is one which has been inserted directly through the abdominal wall into the stomach. It is secured by an internal retention device (either a balloon or a soft disc known as a “bumper”) on the inside and a firm external retention device (known as a “flange”) on the outside.11 Placement of a ballooned gastrostomy tube Cross-section: non-ballooned tube Oesophagus Stomach Clamp External Flange Gastrostomy tube Skin Fat Muscle Skin Internal Bumper Stomach Photo: APhoto: Kennedy Photo: MPhoto: Sutherland Patient with a ballooned gastrostomy Patient lying down with a non-ballooned tube insitu gastrostomy tube in situ See page 8 and 9 for a summary of the different types of tubes and devices you might see. A Clinician’s Guide: Caring for people with gastrostomy tubes and devices 7 Common features of gastrostomy feeding tubes and devices include, but are not limited to: Refer to manufacturer’s guidelines for advice on brand specific tube and device features Ballooned Gastrostomy Tube Ballooned Gastrostomy Tube With side port Without side port Feeding Port Feeding Port (Enteral Dispenser (Enteral Dispenser and Feed Bag and Feed Bag connect here) connect here) ml/cc Balloon Port Balloon Port ml/cc Side Port (X ml/cc) (X ml/cc) French (size) [For example:16/18/20] French (size) [For example:16/18/20] FR FR cm markings cm markings External External Flange Flange Balloon Balloon Non-ballooned Gastrostomy Tube Non-ballooned Gastrostomy Tube with collapsible internal -

General Instructions for Infant and Child Care
Name _______________________________________________ Birth Date ____________________________________________ GENERAL INSTRUCTIONS FOR INFANT AND CHILD CARE GUIDELINES FOR HEALTH EVALUATION VISITS Richmond Pediatrics Pediatric & Adolescent Medicine . for over 50 years 357 N.W. Richmond Beach Road Shoreline, Washington 98177 (206) 546-2421 (206) 546-8436 – Fax www.Richmond-Pediatrics.com Age Immunization Date Given Immunizations >9 Years Old Birth Hepatitis B Immunization Date Given Hepatitis B MCV4 (Meningitis) #1 DtaP MCV4 (Meningitis) #2 IPV (Polio) 2 Months TdaP Hib (Meningitis) HPV #1 PCV13 (Pneumonia) Rotavirus HPV #2 DtaP HPV #3 IPV (Polio) 4 Months Hib (Meningitis) We also recommend a yearly influenza immunization. PCV13 (Pneumonia) Rotavirus Hepatitis B DtaP IPV (Polio) 6 Months Hib (Meningitis) PCV13 (Pneumonia) Rotavirus MMR VZV (Chickenpox) DtaP 12 -18 Hib (Meningitis) Months PCV13 (Pneumonia) Hepatitis A #1 Hepatitis A #2 18mos-4yrs PCV13 booster DtaP IPV 5 Years MMR VZV (Chickenpox) Influenza #1 6mos-5 yrs Influenza #2 TABLE OF CONTENTS Infant Care Page Breast Feeding ..............................................................6 Diarrhea .......................................................................20 Formula .........................................................................8 Dehydration .................................................................20 Feeding Schedule .........................................................9 Fever ...........................................................................22 -

Mic Gastrostomy Feeding Tubes Care Booklet.Pdf
GASTROSTOMY CARE GUIDE GASTROSTOMY CARE GUIDE Universal INDICATIONS FOR Adapter TUBE FEEDING Medication PATIENT INFORMATION Complete nutrition supports Port development, growth, and heal- ing. If the ability to eat or Replaceable Date of tube insertion swallow is lost, or the patient is unable to tolerate food, enteral Feeding feeding can sustain life, nour- Patient name Phone ish, and even increase body Adapter weight. Tube feeding is also Physician Phone used to supplement a deficient food and fluid intake. The feed- Type 0100 0110 0150 0160 (circle one) Fr Size ing procedure can be managed safely and economically at Manufacturer's lot number (printed on package) home, away from the hospital setting. A surgical gastrostomy Mark above the SECUR-LOK® Ring in cm provides access to the stomach if long term nutritional support (this means the mark after the initial placement) is necessary. Balloon volume if 0100, or 0110 type G Tube Pure medical grade silicone (the volume should be between 7 and 10 cc) construction makes MIC Feeding Tubes durable, yet soft and comfortable to wear. They SECUR-LOK® Formula are also translucent, allowing Ring visualization of the inside of Brand name the tube above the skin line. All MIC Enteral Feeding Tubes Method of delivery are latex free. Volume, rate and time the feeding should take MIC PEG Total amount of daily water PEG stands for Percutan- eous (through the skin) Additional ingredients Endoscopic (use of a flexible lighted tube to visualize tube Irrigate the tube with water before and after feeding and medication placement) Gastrostomy administration. (surgical opening into the stomach). -

2Nd Quarter 2001 Medicare Part a Bulletin
In This Issue... From the Intermediary Medical Director Medical Review Progressive Corrective Action ......................................................................... 3 General Information Medical Review Process Revision to Medical Record Requests ................................................ 5 General Coverage New CLIA Waived Tests ............................................................................................................. 8 Outpatient Hospital Services Correction to the Outpatient Services Fee Schedule ................................................................. 9 Skilled Nursing Facility Services Fee Schedule and Consolidated Billing for Skilled Nursing Facility (SNF) Services ............. 12 Fraud and Abuse Justice Recovers Record $1.5 Billion in Fraud Payments - Highest Ever for One Year Period ........................................................................................... 20 Bulletin Medical Policies Use of the American Medical Association’s (AMA’s) Current Procedural Terminology (CPT) Codes on Contractors’ Web Sites ................................................................................. 21 Outpatient Prospective Payment System January 2001 Update: Coding Information for Hospital Outpatient Prospective Payment System (OPPS) ......................................................................................................................... 93 he Medicare A Bulletin Providers Will Be Asked to Register Tshould be shared with all to Receive Medicare Bulletins and health care -

What to Do When Your Child Has the “Tummy Flu”?
What to do when your child has the “Tummy flu”? How can I take care of my child? Vomiting: 1. Diet for breast-fed babies The key to treatment is providing breast milk in smaller amounts than usual. If your baby vomits once, make no changes. If your baby vomits twice, continue breast feeding but nurse on only one side for 10 minutes every 1 to 2 hours. If your baby vomits 3 or more times, nurse for 4 to 5 minutes every 30 to 60 minutes. As soon as 8 have passed without vomiting, return to normal nursing on both sides. Pedialyte is rarely needed for breast-fed babies. If vomiting continues, switch to Pedialyte for 4 hours. Spoon or syringe feed 1 to 2 teaspoons (5 to 10 ml) of Pedialyte every 5 minutes. If your baby is urinating less than normal, you can offer the baby an electrolyte solution between breast-feedings for a short time (6 to 24 hours). 2. Diet for formula-fed babies For infants less than 1 year old, always use an oral electrolyte solution (such as Pedialyte). Spoon or syringe feed your baby 1 teaspoon (5 ml) every 5 minutes. Until you get some Pedialyte, give formula by teaspoon in the same way. 3. If the child is vomiting offer small amount of clear fluids for 8 hours (no solid food) Offer clear fluids (no milk) in small amounts until 8 hours have passed without vomiting. Preferably Pedialyte and/or other oral rehydration formulas. Pedialyte popsicles, Jell-O are other ways to get fluids into the child. -

Expert Mark H
Ask the expert Mark H. DeLegge, Enteral nutrition MD, FASGE 1. Q: How do you code for percutaneous endoscopic jejunostomy (PEJ)? A: For direct (D) PEJ, I use code 44372, with a Relative Value Unit (RVU) of 6.72. Ask the expert features questions submitted 2. Q: In the severely ill patient, is there any role for amino acid supplementation? by members, with A: Arginine and glutamine are two very important amino acids for the critically ill patient, who is often in the intensive care unit (ICU) on a ventilator. Arginine is a conditionally essential amino acid, meaning answers provided by that in critical situations, it should be supplemented. Multiple studies have determined that arginine is an ASGE physician experts. immuno-enhancing supplement that has been shown to improve rates of morbidity and mortality and days ASGE’s Publications on a ventilator in an ICU in some very ill patients. There have been concerns about the use of arginine in septic patients, because arginine is metabolized to nitrous oxide – a vasodilator. However, these concerns Committee identifies are unfounded. authors and topics for Glutamine is an immune-stimulating amino acid that is also trophic for the small bowel mucosa. Although oral the column. In this or enteral administration of glutamine has not been shown to improve clinical outcomes in critically ill patients, issue, Mark H. DeLegge, intravenous administration of glutamine has been shown to be beneficial. Intravenous glutamine is currently available internationally but not in the United States. Therefore, oral arginine supplementation or use of an MD, FASGE, responds arginine-fortified enteral formula should be considered in critically ill patients. -

Approach to Pediatric Vomiting.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Approach to Pediatric Vomiting.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Developed by Erin Boschee and Dr. Melanie Lewis for PedsCases.com. August 25, 2014. Approach to Pediatric Vomiting (Part 1) Introduction Hi, Everyone! My name is Erin Boschee and I’m a medical student at the University of Alberta. This podcast was reviewed by Dr. Melanie Lewis, a General Pediatrician and Associate Professor at the University of Alberta and Stollery Children’s Hospital in Edmonton, Alberta, Canada. This is the first in a series of two podcasts discussing an approach to pediatric vomiting. We will focus on the following learning objectives: 1) Create a differential diagnosis for pediatric vomiting. 2) Highlight the key causes of vomiting specific to the newborn and pediatric population. 3) Develop a clinical approach to pediatric vomiting through history taking, physical exam and investigations. Case Example Let’s start with a case example that we will revisit at the end of the podcasts. You are called to assess a 3-week old male infant for recurrent vomiting and ‘feeding difficulties.’ The ER physician tells you that the mother brought the baby in stating that he started vomiting with every feed since around two weeks of age. In the last three days he has become progressively more sleepy and lethargic. She brought him in this afternoon because he vomited so forcefully that it sprayed her in the face.