The University of New South Wales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
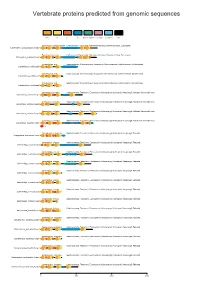
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -

Copyrighted Material
Index INDEX Note: page numbers in italics refer to fi gures, those in bold refer to tables and boxes. abducens nerve 55 activity cycles 499–522 inhibition 485 absorption effi ciency 72 annual patterns 515, 516, 517–22 interactions 485–6 abyssal zone 393 circadian rhythms 505 prey 445 Acanthaster planci (Crown-of-Thorns Starfi sh) diel patterns 499, 500, 501–2, 503–4, reduction 484 579 504–7 aggressive mimicry 428, 432–3 Acanthocybium (Wahoo) 15 light-induced 499, 500, 501–2, 503–4, aggressive resemblance 425–6 Acanthodii 178, 179 505 aglomerular 52 Acanthomorpha 284–8, 289 lunar patterns 507–9 agnathans Acanthopterygii 291–325 seasonal 509–15 gills 59, 60 Atherinomorpha 293–6 semilunar patterns 507–9 osmoregulation 101, 102 characteristics 291–2 supra-annual patterns 515, 516, 517–22 phylogeny 202 distribution 349, 350 tidal patterns 506–7 ventilation 59, 60 jaws 291 see also migration see also hagfi shes; lampreys Mugilomorpha 292–3, 294 adaptive response 106 agnathous fi shes see jawless fi shes pelagic 405 adaptive zones 534 agonistic interactions 83–4, 485–8 Percomorpha 296–325 adenohypophysis 91, 92 chemically mediated 484 pharyngeal jaws 291 adenosine triphosphate (ATP) 57 sound production 461–2 phylogeny 292, 293, 294 adipose fi n 35 visual 479 spines 449, 450 adrenocorticotropic hormone (ACTH) 92 agricultural chemicals 605 Acanthothoraciformes 177 adrianichthyids 295 air breathing 60, 61–2, 62–4 acanthurids 318–19 adult fi shes 153, 154, 155–7 ammonia production 64, 100–1 Acanthuroidei 12, 318–19 death 156–7 amphibious 60 Acanthurus bahianus -

Third International Symposium on Mangroves As Fish Habitat Abstracts*
Bull Mar Sci. 96(3):539–560. 2020 abstracts https://doi.org/10.5343/bms.2019.0047 Third International Symposium on Mangroves as Fish Habitat Abstracts* COMMUNITY COMPOSITION AND DIVERSITY OF PHYTOPLANKTON IN RELATION TO ENVIRONMENTAL VARIABLES AND SEASONALITY IN A TROPICAL MANGROVE ESTUARY, MALAYSIA by ABU HENA MK, Saifullah ASM, Idris MH, Rajaee AH, Rahman MM.—Phytoplankton are the base of the aquatic food chain from which energy is transferred to higher organisms. The community and abundance of phytoplankton in a tropical mangrove estuary were examined in Sarawak, Malaysia. Monthly-collected data from January 2013 to December 2013 was pooled into seasons to examine the influence of seasonality. The estuary was relatively species-rich and a total of 102 species under 43 genera were recorded, comprising 6 species of Cyanophyceae, 4 species of Chlorophyceae, 63 species of Bacillariophyceae, and 29 species of Dinophyceae. The mean abundance (cells L−1) of phytoplankton was found in the following order: Bacillariophyceae > Dinophyceae > Cyanophyceae > Chlorophyceae. Mean abundance of phytoplankton ranged from 5694 to 88,890 cells L−1 over the study period, with a higher value in the dry season. Species recorded from the estuary were dominated by Pleurosigma normanii, Coscinodiscus sp., Coscinodiscus centralis, Coscinodiscus granii, Dinophysis caudata, Ceratium carriense, Ceratium fusus, and Ceratium lineatum. Abundance of phytoplankton was positively influenced by chlorophyll a (R = 0.69), ammonium (R = 0.64), and silica (R = 0.64). Significant differences (ANOSIM and NMDS) were observed in the species community structure between the intermediate and wet season. The species assemblages were positively correlated with surface water temperature, salinity, pH, ammonium, and nitrate in the intermediate and dry season toward larger species composition in the respective seasons, whereas silica influenced species assemblage in the wet season. -

Checklist of Philippine Chondrichthyes
CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 Checklist of Philippine chondrichthyes. Bibliography. ISBN 1 876996 95 1. 1. Chondrichthyes - Philippines. 2. Sharks - Philippines. 3. Stingrays - Philippines. I. Compagno, Leonard Joseph Victor. II. CSIRO. Marine Laboratories. (Series : Report (CSIRO. Marine Laboratories) ; 243). 597.309599 1 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V.1, Last, P.R.2, Stevens, J.D.2, and Alava, M.N.R.3 1 Shark Research Center, South African Museum, Iziko–Museums of Cape Town, PO Box 61, Cape Town, 8000, South Africa 2 CSIRO Marine Research, GPO Box 1538, Hobart, Tasmania, 7001, Australia 3 Species Conservation Program, WWF-Phils., Teachers Village, Central Diliman, Quezon City 1101, Philippines (former address) ABSTRACT Since the first publication on Philippines fishes in 1706, naturalists and ichthyologists have attempted to define and describe the diversity of this rich and biogeographically important fauna. The emphasis has been on fishes generally but these studies have also contributed greatly to our knowledge of chondrichthyans in the region, as well as across the broader Indo–West Pacific. An annotated checklist of cartilaginous fishes of the Philippines is compiled based on historical information and new data. A Taiwanese deepwater trawl survey off Luzon in 1995 produced specimens of 15 species including 12 new records for the Philippines and a few species new to science. -

Malaysia National Plan of Action for the Conservation and Management of Shark (Plan2)
MALAYSIA NATIONAL PLAN OF ACTION FOR THE CONSERVATION AND MANAGEMENT OF SHARK (PLAN2) DEPARTMENT OF FISHERIES MINISTRY OF AGRICULTURE AND AGRO-BASED INDUSTRY MALAYSIA 2014 First Printing, 2014 Copyright Department of Fisheries Malaysia, 2014 All Rights Reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic, mechanical, including photocopy, recording, or any information storage and retrieval system, without prior permission in writing from the Department of Fisheries Malaysia. Published in Malaysia by Department of Fisheries Malaysia Ministry of Agriculture and Agro-based Industry Malaysia, Level 1-6, Wisma Tani Lot 4G2, Precinct 4, 62628 Putrajaya Malaysia Telephone No. : 603 88704000 Fax No. : 603 88891233 E-mail : [email protected] Website : http://dof.gov.my Perpustakaan Negara Malaysia Cataloguing-in-Publication Data ISBN 978-983-9819-99-1 This publication should be cited as follows: Department of Fisheries Malaysia, 2014. Malaysia National Plan of Action for the Conservation and Management of Shark (Plan 2), Ministry of Agriculture and Agro- based Industry Malaysia, Putrajaya, Malaysia. 50pp SUMMARY Malaysia has been very supportive of the International Plan of Action for Sharks (IPOA-SHARKS) developed by FAO that is to be implemented voluntarily by countries concerned. This led to the development of Malaysia’s own National Plan of Action for the Conservation and Management of Shark or NPOA-Shark (Plan 1) in 2006. The successful development of Malaysia’s second National Plan of Action for the Conservation and Management of Shark (Plan 2) is a manifestation of her renewed commitment to the continuous improvement of shark conservation and management measures in Malaysia. -

Epithelial Sodium Channel (Enac) Family: Phylogeny, Structure–Function, Tissue Distribution, and Associated Inherited Diseases
Gene 579 (2016) 95–132 Contents lists available at ScienceDirect Gene journal homepage: www.elsevier.com/locate/gene Gene wiki review Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases Israel Hanukoglu a,⁎, Aaron Hanukoglu b,c a Laboratory of Cell Biology, Faculty of Natural Sciences, Ariel University, Ariel, Israel b Division of Pediatric Endocrinology, E. Wolfson Medical Center, Holon, Israel c Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel article info abstract Article history: The epithelial sodium channel (ENaC) is composed of three homologous subunits and allows the flow of Na+ ions Received 7 September 2015 across high resistance epithelia, maintaining body salt and water homeostasis. ENaC dependent reabsorption of Received in revised form 20 December 2015 Na+ in the kidney tubules regulates extracellular fluid (ECF) volume and blood pressure by modulating osmolar- Accepted 22 December 2015 ity. In multi-ciliated cells, ENaC is located in cilia and plays an essential role in the regulation of epithelial surface Available online 7 January 2016 liquid volume necessary for cilial transport of mucus and gametes in the respiratory and reproductive tracts respectively. Keywords: Ion channels The subunits that form ENaC (named as alpha, beta, gamma and delta, encoded by genes SCNN1A, SCNN1B, Epithelia SCNN1G, and SCNN1D) are members of the ENaC/Degenerin superfamily. The earliest appearance of ENaC Evolution orthologs is in the genomes of the most ancient vertebrate taxon, Cyclostomata (jawless vertebrates) including Transmembrane proteins lampreys, followed by earliest representatives of Gnathostomata (jawed vertebrates) including cartilaginous Kidney sharks. Among Euteleostomi (bony vertebrates), Actinopterygii (ray finned-fishes) branch has lost ENaC genes. -

A Preliminary Global Assessment of the Status of Exploited Marine Fish and Invertebrate Populations
A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS June 30 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria. L.D. Palomares, Rainer Froese, Brittany Derrick, Simon-Luc Nöel, Gordon Tsui Jessika Woroniak Daniel Pauly A report prepared by the Sea Around Us for OCEANA June 30, 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria L.D. Palomares1, Rainer Froese2, Brittany Derrick1, Simon-Luc Nöel1, Gordon Tsui1, Jessika Woroniak1 and Daniel Pauly1 CITE AS: Palomares MLD, Froese R, Derrick B, Nöel S-L, Tsui G, Woroniak J, Pauly D (2018) A preliminary global assessment of the status of exploited marine fish and invertebrate populations. A report prepared by the Sea Around Us for OCEANA. The University of British Columbia, Vancouver, p. 64. 1 Sea Around Us, Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver BC V6T1Z4 Canada 2 Helmholtz Centre for Ocean Research GEOMAR, Düsternbrooker Weg 20, 24105 Kiel, Germany TABLE OF CONTENTS Executive Summary 1 Introduction 2 Material and Methods 3 − Reconstructed catches vs official catches 3 − Marine Ecoregions vs EEZs 3 − The CMSY method 5 Results and Discussion 7 − Stock summaries reports 9 − Problematic stocks and sources of bias 14 − Stocks in the countries where OCEANA operates 22 − Stock assessments on the Sea Around Us website 31 − The next steps 32 Acknowledgements 33 References 34 Appendices I. List of marine ecoregions by EEZ 37 II. Summaries of number of stock by region and 49 by continent III. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

Implementation on International Plan of Action for the Conservation and Management of Sharks
เอกสารวิชาการฉบับที่ ๒/๒๕๕๘ Technical Paper No. 2/2015 การดําเนินการตามแผนปฏิบัติการสากลเพื่อการอนุรักษ5และการบริหารจัดการฉลาม Implementation on International Plan of Action for the Conservation and Management of Sharks จงกลณี แชHมชIาง Chongkolnee Chamchang กรมประมง Department of Fisheries กระทรวงเกษตรและสหกรณ5 Ministry of Agriculture and Cooperatives เอกสารวิชาการฉบับที่ ๒/๒๕๕๘ Technical Paper No. 2/2015 การดําเนินการตามแผนปฏิบัติการสากลเพื่อการอนุรักษ5และการบริหารจัดการฉลาม Implementation on International Plan of Action for the Conservation and Management of Sharks จงกลณี แชHมชIาง Chongkolnee Chamchang กรมประมง Department of Fisheries กระทรวงเกษตรและสหกรณ5 Ministry of Agriculture and Cooperatives ๒๕๕๘ 2015 รหัสทะเบียนวิจัย 58-1600-58130 สารบาญ หนา บทคัดย อ 1 Abstract 2 บัญชีคําย อ 3 บทที่ 1 บทนํา 5 1.1 ความเป#นมาและความสําคัญของป*ญหา 5 1.2 วัตถุประสงค/ของการวิจัย 7 1.3 ขอบเขตของการวิจัย 7 1.4 วิธีดําเนินการวิจัย 7 1.5 ประโยชน/ที่คาดว าจะไดรับ 8 บทที่ 2 แผนปฏิบัติการสากลเพื่อการอนุรักษ/และการบริหารจัดการปลาฉลาม 9 2.1 ที่มาของแผนปฏิบัติการสากล 9 2.2 วัตถุประสงค/ของ IPOA-Sharks 10 2.3 แนวทางปHองกันไวก อน 10 2.4 หลักการพื้นฐานของ IPOA-Sharks 11 2.4.1 ตองการอนุรักษ/ฉลามบางชนิดและปลากระดูกอ อนอื่น ๆ 11 2.4.2 ตองการรักษาความหลากหลายทางชีวภาพโดยการคงไวของประชากรฉลาม 11 2.4.3 ตองการปกปHองถิ่นที่อยู อาศัยของฉลาม 11 2.4.4 ตองการบริหารจัดการทรัพยากรฉลามเพื่อใชประโยชน/อย างยั่งยืน 11 2.5 สาระสําคัญและการปฏิบัติของ IPOA-Sharks 12 2.6 การจัดทําแผนฉลามระดับประเทศ 13 2.7 เนื้อหาแนะนําสําหรับการจัดทําแผนฉลาม 13 2.8 การดําเนินการในระดับภูมิภาคและสถานภาพการจัดทําแผนฉลามของประเทศต -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -
Database of Bibliography of Living/Fossil
www.shark-references.com Version 16.01.2018 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2017 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 DOI: 10.13140/RG.2.2.32409.72801 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 16.01.2018 Abstract: This paper contains a collection of 817 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2017. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2017, list of descriptions of extinct and extant species from 2017, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide data information on the geographic and depth distribution of newly described species, i.e. the type specimens from the years 1990 to 2017 in a hot spot analysis. New in this year's POTY is the subheader "biodiversity" comprising a complete list of all valid chimaeriform, selachian and batoid species, as well as a list of the top 20 most researched chondrichthyan species. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, possible errors cannot entirely be excluded. -

Bos Taurus, Cattle, Cow at Geochembio: Taxonomy, Brief Facts
http://www.GeoChemBio.com: Bos taurus, cattle, cow ● Taxonomy ● Brief facts ● Developmental stages ● Digestive system ● Diagram of digestive system ● Some Bovidae species ● Bovidae species ● Cattle domestication ● Cattle domestication diagram ● Photo gallery Taxonomy cellular organisms - Eukaryota - Fungi/Metazoa group - Metazoa - Eumetazoa - Bilateria - Coelomata - Deuterostomia - Chordata - Craniata - Vertebrata - Gnathostomata - Teleostomi - Euteleostomi - Sarcopterygii - Tetrapoda - Amniota - Mammalia - Theria - Eutheria - Laurasiatheria - Cetartiodactyla - Ruminantia - Pecora - Bovidae - Bovinae - Bos - Bos taurus The two principal taxonomic groups of domestic cattle are Bos taurus (taurine cattle) and Bos indicus (zebu cattle). General description ● Domestic cows are common throughout the world. They are grown and bred for food - meat and milk production, as well as for working - plowing and moving heavy loads. ● Domestic cows are social animals and live in herds which are structured according to a dominance hierarchy. ● Cows feed on grasses and other herbaceous plants. An average cow can consume about 70 kg of grass in an 8 hour day. Cows are ruminants. They have a four chambered stomach. Cow's milk allergy vs. cow's milk intolerance ● Cow's milk allergy and cow's milk intolerance are two different concepts that are used interchangeably. The former is an immunologically mediated reaction to the milk's contents, not unlike to any other allergic reactions, for example, to eggs, corn, nuts, etc. The latter is non-immunologic reaction to the cow's milk, the most common cause of which is deficiency of lactase - the enzyme that breaks down one of the main nutrients of the milk - lactose. Importance of bovine genome ● The bovine genome serves as a reference non-primate, non-rodent, eutherian genome.