Gas Density Is That of Oxygen Since It Is Commonly Employed As the Standard Gas in the Application of Avogadro's Rule
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Revision Guide
Revision Guide Chemistry - Unit 3 Physical and Inorganic Chemistry GCE A Level WJEC These notes have been authored by experienced teachers and are provided as support to students revising for their GCE A level exams. Though the resources are comprehensive, they may not cover every aspect of the specification and do not represent the depth of knowledge required for each unit of work. 1 Content Page Section 2 3.1 – Redox and standard electrode potential 13 3.2 - Redox reactions 20 3.3 - Chemistry of the p-block 30 3.4 - Chemistry of the d-block transition metals 35 3.5 - Chemical kinetics 44 3.6 - Enthalpy changes for solids and solutions 50 3.7 - Entropy and feasibility of reactions 53 3.8 - Equilibrium constants 57 3.9 - Acid-base equilibria 66 Acknowledgements 2 3.1 – Redox and standard electrode potential Redox reactions In AS, we saw that in redox reactions, something is oxidised and something else is reduced (remember OILRIG – this deals with loss and gain of electrons). Another way that we can determine if a redox reaction has happened is by using oxidation states or numbers (see AS revision guide pages 2 and 44). You need to know that: - • oxidation is loss of electrons • reduction is gain of electrons • an oxidising agent is a species that accepts electrons, thereby helping oxidation. It becomes reduced itself in the process. • a reducing agent is a species that donates electrons, thereby helping reduction. It becomes oxidised itself in the process. You also should remember these rules for assigning oxidation numbers in a compound: - 1 All elements have an oxidation number of zero (including diatomic molecules like H2) 2 Hydrogen is 1 unless it’s with a Group 1 metal, then it’s -1 3 Oxygen is -2 (unless it’s a peroxide when it’s -1, or reacted with fluorine, when it’s +2). -
A Manganate Ester Hydrolysis Mn(IV)
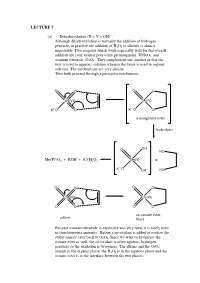
LECTURE 7 (a) Dihydroxylation (X = Y = OH) Although dihydroxylation is formally the addition of hydrogen peroxide, in practice the addition of H2O2 to alkenes is almost impossible. Two reagents which work especially well for this overall addition are cold, neutral potassium permanganate, KMnO4, and osmium tetroxide, OsO4. They complement one another in that the first is used in aqueous solution whereas the latter is used in organic solvents. The mechanisms are very similar. Thus both proceed through a pericyclic mechanism: O O O O Mn(VII) Mn(V) +- K+-O O K O O a manganate ester hydrolysis O OH HO Mn(IV)O2 + KOH + 0.5 H2O2 Mn(V) + +- K O OH HO O O O O Os(VIII) Os(VI) O O O O an osmate ester, yellow black Because osmium tetroxide is expensive and very toxic it is rarely used in stoichiometric amounts. Rather a co-oxidant is added to oxidise the stable osmate ester back to OsO4. Since we want to hydrolyse the osmate ester as well, the co-oxidant is often aqueous hydrogen peroxide so the oxidation is two-phase. The alkene and the OsO4 remain in the organic phase, the H2O2 is in the aqueous phase and the osmate ester is at the interface between the two phases: H OH H OH H OsO4, + aq.H2O2, CH Cl H 2 2 OH H H OH (c) Epoxidation (X = Y = O) The reaction of alkenes with peroxy acids (RCO3H) leads to a cyclic ether, known as an epoxide, as follows: R R O O O H O H O O Although the reaction will work with peroxyacetic acid (R = Me) it works best with peroxy acids bearing electron-withdrawing groups e.g. -

The Anodic Oxidation of Maleic Acid
Scholars' Mine Masters Theses Student Theses and Dissertations 1966 The anodic oxidation of maleic acid Larry D. Gilmartin Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Chemical Engineering Commons Department: Recommended Citation Gilmartin, Larry D., "The anodic oxidation of maleic acid" (1966). Masters Theses. 5776. https://scholarsmine.mst.edu/masters_theses/5776 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. THE ANODIC OXIDATION OF MALEIC ACID BY LARRY D. GILMARTIN A THESIS submitted to the faculty of THE UNIVERSITY OF MISSOURI AT ROLLA in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE IN CHEMICAL ENGINEERING Rolla, Missouri 1966 Approved by (advisor) THE ANODIC OXIDATION OF MALEIC ACID Larry D. Gilmartin ABSTRACT The purpose of this investigation was to determine the mechanism of the anodic oxidation of maleic acid on platinized-platinum electrodes at 80°C. Current density-potential studies were conducted varying the parameters of maleic acid concentration and pH. The faradaic efficiency of the oxidation of maleic acid to yield co 2 was determined. The effect of temperature on current density was also studied to determine the activation energy for the reaction. The oxidation of maleic acid occurred only in acidic solutions. The faradaic efficiency was found to be ap proximately 97 ± 5 per cent. A linear Tafel region was found which had a slope of 145 - 170 millivolts <~ 2.3RT/aF). -

Complexes of Ferrous Iron with Tannic Acid Fy J
Complexes of Ferrous Iron With Tannic Acid fy J. D. HEM :HEMISTRY OF IRON IN NATURAL WATER GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1459-D IITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1960 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. CONTENTS Page Abstract. _________________________________________________________ 75 Acknowledgments. ________________________________________________ 75 Organic complexing agents________-______-__-__-__-______-____-___-- 75 Tannic acid_______________________________________________________ 77 Properties ____________________________________________________ 78 Dissociation._________________________________________________ 78 Reducing action_____--_-______________________________________ 79 Laboratory studies_______________________________________________ 79 Analytical procedures__________________________________________ 80 Chemical reactions in test solutions._____________________________ 81 No tannic acid____________________-_________________-_--__ 84 Five parts per million of tannic acid- ________________________ 84 Fifty parts per million of tannic acid_____-________-____------ 85 Five hundred parts per million of tanni c acid _________________ 86 Rate of oxidation and precipitation of iron______________________ 87 Stability constants for tannic acid complexes______________________ 88 Comparison of determined and estimated Eh______________________ -

Process Development for Permanganate Addition During Oxidative Leaching of Hanford Tank Sludge Simulants
PNNL-16794 WTP-RPT-164, Rev 0 Process Development for Permanganate Addition During Oxidative Leaching of Hanford Tank Sludge Simulants B. M. Rapko G. J. Lumetta J. R. Deschane R. A. Peterson October 2007 Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor Battelle Memorial Institute, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or Battelle Memorial Institute. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. PACIFIC NORTHWEST NATIONAL LABORATORY operated by BATTELLE for the UNITED STATES DEPARTMENT OF ENERGY under Contract DE-ACO5-76RL01830 PNNL-16794 WTP-RPT-164, Rev 0 Process Development for Permanganate Addition During Oxidative Leaching of Hanford Tank Sludge Simulants B. M. Rapko G. J. Lumetta J. R. Deschane R. A. Peterson October 2007 Test specification: 24590-PTF-TSP-RT-06-002, Rev. 0 Test Plan: TP-RPP-WTP-453, Rev. 0, and ICN-TP-RPP-WTP-453.1 Test exceptions: 24590-WTP-TEF-RT-07-00002 R&T focus area: Pretreatment Test scoping statement(s): None Pacific Northwest National Laboratory Richland, Washington 99352 Contents Acronyms.................................................................................................................................................... -

Oxidative Kinetic Study of Fluoroquinolone Pharmaceuticals in Acidic/Basic Aqueous Solutions
OXIDATIVE KINETIC STUDY OF FLUOROQUINOLONE PHARMACEUTICALS IN ACIDIC/BASIC AQUEOUS SOLUTIONS A THESIS Submitted to the University of Kota, Kota for the Degree Of Doctor of Philosophy In Chemistry (Faculty of Science) Submitted by: ANKITA JAIN Under the Supervision of Dr. (Mrs.) Vijay Devra Department of Chemistry J. D. B. Govt. P.G. Girls College Kota (Rajasthan) 2017 Dedicated to My Parents, My Husband Amit & My Daughter Aarna University of Kota, Kota M. B. S. Marg, Near Kabir Circle, Rawatbhata Road, Kota (Raj.) Certificate It is to certify that, (i) The thesis entitled “Oxidative Kinetic Study of Fluoroquinolone Pharmaceuticals in Acidic/Basic Aqueous Solutions” submitted by Ankita Jain is an original piece of research work carried out by the candidate under my supervision. (ii) Literary presentation is satisfactory and the thesis is in a form suitable for publication. (iii) Work evidences the capacity of the candidate for critical examination and independent judgment. (iv) Fulfills the requirement of the ordinance relating to the Ph.D. degree of the university. (v) Candidate has put in at least 200 days of attendance every year. Dr. (Mrs.) Vijay Devra Department of Chemistry, J. D. B. Govt. P. G. Girls College, Kota (Raj.) A Word of Gratitude “Words are powerless to express my gratitude.” I take this opportunity to express my deep sense of gratitude to my adored mentor and supervisor Dr. (Mrs.) Vijay Devra, Senior Lecturer, J. D. B. Govt. Girls College, Kota, (Raj.) for her constant guidance, cooperation, motivation and support. Without her kind and patient instruction, it is impossible for me to finish this thesis. -

Manganese Oxide Minerals: Crystal Structures and Economic and Environmental Significance
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3447–3454, March 1999 Colloquium Paper This paper was presented at the National Academy of Sciences colloquium ‘‘Geology, Mineralogy, and Human Welfare,’’ held November 8–9, 1998 at the Arnold and Mabel Beckman Center in Irvine, CA. Manganese oxide minerals: Crystal structures and economic and environmental significance JEFFREY E. POST Department of Mineral Sciences, Smithsonian Institution, Washington, DC 20560-0119 ABSTRACT Manganese oxide minerals have been used ronmentally relevant insights into certain types of interactions for thousands of years—by the ancients for pigments and to between these systems and potentially serve as long-term clarify glass, and today as ores of Mn metal, catalysts, and monitors of changes within a system. battery material. More than 30 Mn oxide minerals occur in a As ores, Mn oxides have been exploited since ancient times. wide variety of geological settings. They are major components In particular, pyrolusite (MnO2) was prized as a pigment and of Mn nodules that pave huge areas of the ocean floor and for its ability to remove the green tint imparted by iron to glass bottoms of many fresh-water lakes. Mn oxide minerals are (3). By the mid-19th century Mn was an essential component ubiquitous in soils and sediments and participate in a variety in steel making, as a deoxidizer and desulfurizer and for of chemical reactions that affect groundwater and bulk soil making hard-steel alloys. Mn oxides are the predominant ore composition. Their typical occurrence as fine-grained mix- minerals in most of today’s commercially important Mn de- tures makes it difficult to study their atomic structures and posits, commonly formed by weathering of Mn-rich carbonates crystal chemistries. -

The Reaction of Barium Manganate with Acids and Their Precursors
Indian Journal of Chemistry Vol. 38A. September 1999, pp.966-968 The reaction of barium manganate with fraction data were collected on a Phillips PW 3710 acids and their precursors diffractometer, with a Cu monochromator. Synthesis oj barium manganate (VI) Liszlo Kotai, Agnes Keszler, Janos Pato. Sandor Holly Chemical Research Center, Institute of Chemistry. KMn04 (15.8 g) was dissolved in 300 ml of water, Hungarian Academy of Sciences then BaCI2.2Hp (24.9 g dissolved in 100 ml of water), H-1025, Budapest, Pusztaseri u. 59-67, Hungary KOH (56 g dissolved in 100 ml of water) and KI (2.0 g and dissolved in 20 ml of water) were added with vigorous Kalyan K Banerji ' Department of Chemistry, J N V University, stirring. The mixture was boiled for 15 min, cooled, Jodhpur 342 005, India filtered, and washed. The permanganate-free product was dried at 105° C for I h, then the traces of water were Received 30 November 1998; revised 4 Mal' 1999 removed by azeotropic distillation with benzene (yield - 100%). Analysis (found/calc. fo r BaMn0 ): Ba 53.69/ 4 A simple and easy preparative route to obtain permangani c ac id 53.59%; Mn 21.40121.44%. and permanganate salts from barium manganate and sulphuric acid is described. Sulphuric acid reacts with bari um manganate to pro duce sparingly soluble bariulll sulphate and well-soluble permanga Synthesis ojpermanganic acid nic ac id or bariulll permanganate, these in turn can be usee! to pre To barium manganate (2.56 g, 0 .0 I mol) suspended pare ot her metal permanganates. -

Conversion of Manganese Dioxide to Permanganate
Europaisches Patentamt 0 336 542 I � European Patent Office © Publication number: A1 Office europeen des brevets 2) EUROPEAN PATENT APPLICATION S) Application number: 89301730.1 © int. CI.4: C01G 45/12 , C25B 1/28 g) Date of filing: 22.02.89 §) Priority: 09.03.88 US 165752 © Applicant: MACDERMID INCORPORATED 50 Brookside Road S) Date of publication of application: Waterbury Connecticut 06702(US) 11.10.89 Bulletin 89/41 @ Inventor: D'ambrisi, Joseph J. © Designated Contracting States: 32 Tanager Lane CH DE ES FR GB IT LI NL SE Trumbull Connecticut 06611 (US) © Representative: Bankes, Stephen Charles Digby et al BARON & WARREN 18 South End Kensington London W8 5BU(GB) © Conversion of manganese dioxide to permanganate. © A process is described for the electrolytic oxidation of manganese dioxide to an alkali metal permanganate by carrying out the electrolysis in dilute alkali metal hydroxide solution (2) using a non-sacrificial anode (6) and a cathode (10) comprising an alkaliresistant electrode (12) immersed in concentrated alkali metal hydroxide solution (16) in a porous container (18). The process is particularly adapted to the regeneration of alkali metal permanganate from manganese dioxide which has been precipitated during use of a permanganate bath as an etchant in the fabrication of printed circuit boards and like purposes. CM LO CO CO CO LU Xerox Copy Centre EP 0 336 542 A1 CONVERSION OF MANGANESE DIOXIDE TO PERMANGANATE This invention relates to the conversion of manganese dioxide to alkali metal permanganate and is more particularly concerned with the electrolytic oxidation of manganese dioxide to an alkali metal permanganate and the regeneration of permanganate etchant baths. -

The Impact of Potassium Manganate (Vii)
CIVIL AND ENVIRONMENTAL ENGINEERING REPORTS ISSN 2080-5187 CEER 2017; 27 (4): 029-041 DOI: 10.1515/ceer-2017-0048 Original Research Article THE IMPACT OF POTASSIUM MANGANATE (VII) ON THE EFFECTIVENESS OF COAGULATION IN THE REMOVAL OF IRON AND MANGANESE FROM GROUNDWATER WITH AN INCREASED CONTENT OF ORGANIC SUBSTANCES Izabela KRUPIŃSKA1 University of Zielona Góra, Zielona Góra, Poland A b s t r a c t The article presents the results of studies concerning the impact of the method of Fe(II) ion oxidisation (dissolved oxygen and potassium manganate (VII)) on the effectiveness of coagulation in the removal of iron and manganese from groundwater with an increased content of organic substances. The efficiencies of two coagulants were compared: aluminium sulphate (VI) and polyaluminium chloride (Flokor 1.2A). Among the used methods of iron (II) oxidisation, the best effects have been achieved by potassium manganate (VII) because one of the oxidation products was manganese oxide (IV) precipitating from water. Better results in purifying the water were obtained with the use of a prehydrolysed coagulant Flokor 1.2 A than aluminium sulphate (VI). Keywords: groundwater, iron, manganese, organic substances, potassium manganate (VII), coagulation 1. INTRODUCTION Traditional air oxidation in water treatment is used for the removal of divalent forms of iron and manganese for public health purposes. These ions are frequently present in groundwaters with low oxygen content [22, 28, 29]. 1 Corresponding author: University of Zielona Gora, Faculty of Civil and Environmental Engineering Institute of Environmental Engineering, Szafrana st 15, 65-246 Zielona Góra, Poland, e-mail: [email protected] tel.