Complexes of Ferrous Iron with Tannic Acid Fy J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tannic Acid-Based Prepolymer Systems for Enhanced Intumescence in Epoxy Thermosets
Cite this article Themed Issue: Sustainable flame Keywords: environmental impact/green Korey M, Johnson A, Webb W et al. (2020) retarded materials polymers/sustainable materials Tannic acid-based prepolymer systems for enhanced intumescence in epoxy thermosets. Paper 1900061 Green Materials 8(3): 150–161, Received 29/09/2019; Accepted 05/03/2020 https://doi.org/10.1680/jgrma.19.00061 Published online 06/04/2020 ICE Publishing: All rights reserved Green Materials Tannic acid-based prepolymer systems for enhanced intumescence in epoxy thermosets Matthew Korey Mark Dietenberger Graduate Research Assistant, Purdue University, West Lafayette, IN, USA Research General Engineer, Forest Products Laboratory, Madison, WI, USA (Orcid:0000-0002-2285-5646) Jeffrey Youngblood Alexander Johnson Professor, Purdue University, West Lafayette, IN, USA Undergraduate Research Assistant, Purdue University, West Lafayette, IN, USA (Orcid:0000-0002-8720-8642) William Webb John Howarter Staff, Career Academy, San Diego, CA, USA Associate Professor, Purdue University, West Lafayette, IN, USA (corresponding author: [email protected]) Tannic acid (TA) is a bio-based high-molecular-weight organic molecule. Although biologically sourced, TA is a pollutant in industrial wastewater streams, and there is desire to find applications in which to downcycle this molecule. Many flame retardants (FRs) used in epoxy are synthesized from petroleum-based monomers. Various bio-based modifiers have been developed, but increasing the flame retardancy of the system without trade-offs with other properties has proved challenging. In this work, TA is incorporated into the thermoset. The molecular behavior of the system was dependent on the TA loading, with low concentrations causing the molecule to be surface-functionalized, while at higher concentrations the molecule was cross-linked into the network. -

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

The Use of Plants in the Traditional Management of Diabetes in Nigeria: Pharmacological and Toxicological Considerations
Journal of Ethnopharmacology 155 (2014) 857–924 Contents lists available at ScienceDirect Journal of Ethnopharmacology journal homepage: www.elsevier.com/locate/jep Review The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations Udoamaka F. Ezuruike n, Jose M. Prieto 1 Center for Pharmacognosy and Phytotherapy, Department of Pharmaceutical and Biological Chemistry, School of Pharmacy, University College London, 29-39 Brunswick Square, WC1N 1AX London, United Kingdom article info abstract Article history: Ethnopharmacological relevance: The prevalence of diabetes is on a steady increase worldwide and it is Received 15 November 2013 now identified as one of the main threats to human health in the 21st century. In Nigeria, the use of Received in revised form herbal medicine alone or alongside prescription drugs for its management is quite common. We hereby 26 May 2014 carry out a review of medicinal plants traditionally used for diabetes management in Nigeria. Based on Accepted 26 May 2014 the available evidence on the species' pharmacology and safety, we highlight ways in which their Available online 12 June 2014 therapeutic potential can be properly harnessed for possible integration into the country's healthcare Keywords: system. Diabetes Materials and methods: Ethnobotanical information was obtained from a literature search of electronic Nigeria databases such as Google Scholar, Pubmed and Scopus up to 2013 for publications on medicinal plants Ethnopharmacology used in diabetes management, in which the place of use and/or sample collection was identified as Herb–drug interactions Nigeria. ‘Diabetes’ and ‘Nigeria’ were used as keywords for the primary searches; and then ‘Plant name – WHO Traditional Medicine Strategy accepted or synonyms’, ‘Constituents’, ‘Drug interaction’ and/or ‘Toxicity’ for the secondary searches. -

Biomolecules
biomolecules Article Tannic Acid Improves Renal Function Recovery after Renal Warm Ischemia–Reperfusion in a Rat Model Louise Alechinsky 1, Frederic Favreau 2,3, Petra Cechova 4 , Sofiane Inal 1,5, Pierre-Antoine Faye 2,3, Cecile Ory 1, Raphaël Thuillier 1,5,6,7 , Benoit Barrou 1, Patrick Trouillas 8, Jerome Guillard 9 and Thierry Hauet 1,5,6,7,* 1 INSERM, U1082 IRTOMIT, 86021 Poitiers, France; [email protected] (L.A.); sofi[email protected] (S.I.); [email protected] (C.O.); [email protected] (R.T.); [email protected] (B.B.) 2 Université de Limoges, Faculté de Médecine, EA 6309 “Maintenance Myélinique et Neuropathies Périphériques”, 87025 Limoges, France; [email protected] (F.F.); [email protected] (P.-A.F.) 3 CHU de Limoges, Laboratoire de Biochimie et Génétique Moléculaire, 87042 Limoges, France 4 University Palacký of Olomouc, RCPTM, Dept Physical Chemistry, Faculty of Science, 771 46 Olomouc, Czech Republic; [email protected] 5 CHU de Poitiers, Laboratoire de Biochimie, 86021 Poitiers, France 6 Université de Poitiers, Faculté de Médecine et de Pharmacie, 86073 Poitiers, France 7 Département Hospitalo-Universitaire de Transplantation SUPORT, 86021 Poitiers, France 8 Inserm, UMR 1248, Fac. Pharmacy, Univ. Limoges, 87025 Limoges, France; [email protected] 9 Université de Poitiers, UMR CNRS 7285 IC2MP, Team 5 Organic Chemistry, 86073 Poitiers, France; [email protected] * Correspondence: [email protected] Received: 11 February 2020; Accepted: 9 March 2020; Published: 12 March 2020 Abstract: Background and purpose: Ischemia–reperfusion injury is encountered in numerous processes such as cardiovascular diseases or kidney transplantation; however, the latter involves cold ischemia, different from the warm ischemia found in vascular surgery by arterial clamping. -

Conductometric Study of the Acidity Properties of Tannic Acid (Chinese Tannin)
Journal of the UniversityL. Costadinnova, of Chemical M. Hristova, Technology T. Kolusheva, and Metallurgy, N. Stoilova 47, 3, 2012, 289-296 CONDUCTOMETRIC STUDY OF THE ACIDITY PROPERTIES OF TANNIC ACID (CHINESE TANNIN) L. Costadinnova1, M. Hristova1, T. Kolusheva1, N. Stoilova2 1 University of Chemical Technology and Metallurgy Received 22 May 2012 8 Kl. Ohridski, 1756 Sofia, Bulgaria Accepted 12 June 2012 2 CLVCE, Department of VMP Analysis, 5 Iskarsko shose Blvd., Sofia, Bulgaria E-mail: [email protected] ABSTRACT Two tannic acids are studied (H T, n = 52), C H O , with average molar mass 1701.20 g mol-1. Using their UV and n 76 52 46 IR spectra it is shown that they have identical composition with respect to their functional groups, while by potentiometric and conductometric titration their structure of chinese tannin is verified and the relations between the acidity constants K > K K ... are determined. The absence of gallic acid is proved by HPLC. The conformational flexibility of the a1 a2 : a3 : tannin molecule is used to measure the stepwise constant K . By direct conductometry the acids were studied in the a1 concentration range of 5.00x10-4 to 5.00x10-2 mol l-1. The latter is determined from the Onsager-Shedlovsky relation. The molar conductivity of the ions − for the infinitely dilute solutions of the two tannic acids is found to be 55.2 and HTn1− 64.3 S L mol-1 cm-1. The degree of dissociation á in the studied concentration range varies from 0.03 to 0.3. The results for the acidity constant exponent pK are generalised using variance analysis, yielding ±∆ = ± , n = a1 pKa1 pK a1 4.19 0.02 26. -

Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone
International Journal of Molecular Sciences Article Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone Viktor A. Timoshnikov 1,*, Tatyana V. Kobzeva 1, Nikolay E. Polyakov 1 and George J. Kontoghiorghes 2,* 1 Institute of Chemical Kinetics & Combustion, 630090 Novosibirsk, Russia; [email protected] (T.V.K.); [email protected] (N.E.P.) 2 Postgraduate Research Institute of Science, Technology, Environment and Medicine, CY-3021 Limassol, Cyprus * Correspondence: [email protected] (V.A.T.); [email protected] (G.J.K.); Tel./Fax: +7-383-3332947 (V.A.T.); +357-2627-2076 (G.J.K.) Received: 21 February 2020; Accepted: 28 May 2020; Published: 31 May 2020 Abstract: Ascorbic acid (AscH2) is one of the most important vitamins found in the human diet, with many biological functions including antioxidant, chelating, and coenzyme activities. Ascorbic acid is also widely used in medical practice especially for increasing iron absorption and as an adjuvant therapeutic in iron chelation therapy, but its mode of action and implications in iron metabolism and toxicity are not yet clear. In this study, we used UV–Vis spectrophotometry, NMR spectroscopy, and EPR spin trapping spectroscopy to investigate the antioxidant/pro-oxidant effects of ascorbic acid in reactions involving iron and the iron chelator deferiprone (L1). The experiments were carried out in a weak acidic (pH from 3 to 5) and neutral (pH 7.4) medium. Ascorbic acid exhibits predominantly pro-oxidant activity by reducing Fe3+ to Fe2+, followed by the formation of dehydroascorbic acid. As a result, ascorbic acid accelerates the redox cycle Fe3+ Fe2+ in the Fenton reaction, which leads $ to a significant increase in the yield of toxic hydroxyl radicals. -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Revision Guide
Revision Guide Chemistry - Unit 3 Physical and Inorganic Chemistry GCE A Level WJEC These notes have been authored by experienced teachers and are provided as support to students revising for their GCE A level exams. Though the resources are comprehensive, they may not cover every aspect of the specification and do not represent the depth of knowledge required for each unit of work. 1 Content Page Section 2 3.1 – Redox and standard electrode potential 13 3.2 - Redox reactions 20 3.3 - Chemistry of the p-block 30 3.4 - Chemistry of the d-block transition metals 35 3.5 - Chemical kinetics 44 3.6 - Enthalpy changes for solids and solutions 50 3.7 - Entropy and feasibility of reactions 53 3.8 - Equilibrium constants 57 3.9 - Acid-base equilibria 66 Acknowledgements 2 3.1 – Redox and standard electrode potential Redox reactions In AS, we saw that in redox reactions, something is oxidised and something else is reduced (remember OILRIG – this deals with loss and gain of electrons). Another way that we can determine if a redox reaction has happened is by using oxidation states or numbers (see AS revision guide pages 2 and 44). You need to know that: - • oxidation is loss of electrons • reduction is gain of electrons • an oxidising agent is a species that accepts electrons, thereby helping oxidation. It becomes reduced itself in the process. • a reducing agent is a species that donates electrons, thereby helping reduction. It becomes oxidised itself in the process. You also should remember these rules for assigning oxidation numbers in a compound: - 1 All elements have an oxidation number of zero (including diatomic molecules like H2) 2 Hydrogen is 1 unless it’s with a Group 1 metal, then it’s -1 3 Oxygen is -2 (unless it’s a peroxide when it’s -1, or reacted with fluorine, when it’s +2). -

Hplc∓Dad∓ESI-MS/MS Screening of Bioactive Components
Food Chemistry 166 (2015) 179–191 Contents lists available at ScienceDirect Food Chemistry journal homepage: www.elsevier.com/locate/foodchem HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits ⇑ Ibrahim M. Abu-Reidah a,b,c, Mohammed S. Ali-Shtayeh a, , Rana M. Jamous a, David Arráez-Román b,c, ⇑ Antonio Segura-Carretero b,c, a Biodiversity & Environmental Research Center (BERC), Til, Nablus POB 696, Palestine b Department of Analytical Chemistry, Faculty of Sciences, University of Granada, Avda. Fuentenueva, 18071 Granada, Spain c Functional Food Research and Development Centre (CIDAF), PTS Granada, Avda. del Conocimiento, Edificio Bioregión, 18016 Granada, Spain article info abstract Article history: Rhus coriaria L. (sumac) is an important crop widely used in the Mediterranean basin as a food spice, and Received 25 March 2014 also in folk medicine, due to its health-promoting properties. Phytochemicals present in plant foods are in Received in revised form 29 May 2014 part responsible for these consequent health benefits. Nevertheless, detailed information on these Accepted 3 June 2014 bioactive compounds is still scarce. Therefore, the present work was aimed at investigating the Available online 12 June 2014 phytochemical components of sumac fruit epicarp using HPLC–DAD–ESI-MS/MS in two different ionisation modes. The proposed method provided tentative identification of 211 phenolic and other Keywords: phyto-constituents, most of which have not been described so far in R. coriaria fruits. More than 180 Palestinian sumac phytochemicals (tannins, (iso)flavonoids, terpenoids, etc.) are reported herein in sumac fruits for the first Anacardiaceae Hydrolysable tannins time. -

BARNES-DISSERTATION-2016.Pdf (1.557Mb)
ABSORPTION AND METABOLISM OF MANGO (MANGIFERA INDICA L.) GALLIC ACID AND GALLOYL GLYCOSIDES A Dissertation by RYAN CRISPEN BARNES Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Stephen Talcott Co-Chair of Committee, Susanne Talcott Committee Members, Nancy Turner Arul Jayaraman Head of Department, Boon Chew December 2016 Major Subject: Food Science and Technology Copyright 2016 Ryan Barnes ABSTRACT The composition, absorption, metabolism, and excretion of gallic acid, monogalloyl glucose, and gallotannins in mango (Mangifera indica L.) pulp were investigated. Each galloyl derivative was hypothesized to have a different rate of absorption, and their concentrations were compared in the pulp of five mango varieties. The cultivar Ataulfo was found to have the highest concentration of monogalloyl glucose and gallotannins while the cultivar Kent had the lowest. Enzymatic hydrolysis of gallotannins with tannase led to the characterization of six digalloyl glucoses and five trigalloyl glucoses that have the potential to be formed in the colon following gallotannin consumption. The bioaccessibility of galloyl derivatives was evaluated in both homogenized mango pulp and 0.65 mm3 cubes following in vitro digestion conditions. Monogalloyl glucose was found to be bioaccessible in both homogenized and cubed mango pulp. However, cubed mango pulp had a significantly higher amount of gallotannins still bound to the fruit following digestion. Gallic acid bioaccessibility significantly increased following digestion in both homogenized and cubed mango pulp, likely from hydrolysis of gallotannins. Additionally, for the first time, the absorption of monogalloyl glucose and gallic acid was investigated in both Caco-2 monolayer transport models and a porcine pharmacokinetic model with no significant differences found in their absorption or ability to produce phase II metabolites. -

VCI Powder Corrosion Inhibitors
VCI POWDERS FOR CORROSION PREVENTION ARE IDEAL FOR SHORT TERM LAY UP AND LONG TERM CORROSION PROTECTION OF TANKS, COOLING TOWERS, VESSELS AND ENCLOSED SPACES Vci powders can also be used in water for • Hydro Static Testing and test stands including cast iron • A flush through application of corrosion inhibitors • A final rinse additive for corrosion control in production lines cleaning and protecting metal parts Ideal for hard to reach areas and the protection of: • Tanks • Pipes • Lay Up of Plant & Equipment • Lay up of cooling towers • Preservation of boilers, tubes and condensers • Protection of metal parts and equipment inside any enclosed container * Additive to shot blast, hydro test and metalworking solutions for corrosion control * Corrosion Protection VCI Powder Features: • Corrosion protection in the contact and vapor phase • Vapor Corrosion Inhibitor (VCI) provides a component that is attracted to all metal surfaces and forms a molecular barrier that provides protection from rust and corrosion • Nitrite, Silica and heavy metals free • Environmentally Friendly • Non-hazardous • Easy to Use • Economical Choose from the following types of VCI corrosion control powders available in 5 pound, 50 pound or 100 pound quantities: VCI-1 Powder • Corrosion protection for ferrous metals and aluminum • Apply by dry fogging, spray solution or sprinkling • Use as a hydrotesting additive to water @ ½ to 2 % percent by weight • Great for preservation of plant, cooling towers and equipment • 100% biodegradable and non-hazardous VCI Powder 1010 • Provides continuous long and short-term corrosion protection for ferrous metals and is compatible with copper, brass, zinc and galvanized steel • Water soluable up to 10 % in water by weight • Includes all of the features of VCI Powder 1 and is also compatible with non-ferrous metals Distributed by: KPR ADCOR INC. -
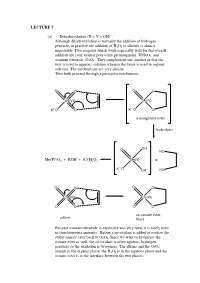
A Manganate Ester Hydrolysis Mn(IV)
LECTURE 7 (a) Dihydroxylation (X = Y = OH) Although dihydroxylation is formally the addition of hydrogen peroxide, in practice the addition of H2O2 to alkenes is almost impossible. Two reagents which work especially well for this overall addition are cold, neutral potassium permanganate, KMnO4, and osmium tetroxide, OsO4. They complement one another in that the first is used in aqueous solution whereas the latter is used in organic solvents. The mechanisms are very similar. Thus both proceed through a pericyclic mechanism: O O O O Mn(VII) Mn(V) +- K+-O O K O O a manganate ester hydrolysis O OH HO Mn(IV)O2 + KOH + 0.5 H2O2 Mn(V) + +- K O OH HO O O O O Os(VIII) Os(VI) O O O O an osmate ester, yellow black Because osmium tetroxide is expensive and very toxic it is rarely used in stoichiometric amounts. Rather a co-oxidant is added to oxidise the stable osmate ester back to OsO4. Since we want to hydrolyse the osmate ester as well, the co-oxidant is often aqueous hydrogen peroxide so the oxidation is two-phase. The alkene and the OsO4 remain in the organic phase, the H2O2 is in the aqueous phase and the osmate ester is at the interface between the two phases: H OH H OH H OsO4, + aq.H2O2, CH Cl H 2 2 OH H H OH (c) Epoxidation (X = Y = O) The reaction of alkenes with peroxy acids (RCO3H) leads to a cyclic ether, known as an epoxide, as follows: R R O O O H O H O O Although the reaction will work with peroxyacetic acid (R = Me) it works best with peroxy acids bearing electron-withdrawing groups e.g.