Carbonyl Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nomenclature of Carboxylic Acid Derivatives Acid Halide Substituents
Gentilucci, Carboxylic Acid Derivatives Nomenclature of Carboxylic Acid Derivatives Gentilucci, Carboxylic Acid Derivatives Acid halides 1. Alkane + the suffix -oyl followed by the halogen. 2. Select the longest continuous carbon chain, containing the acyl group. 3. Number the carbon chain, beginning at the end nearest to the acyl group. 4. Number the substituents and write the name, listing substituents alphabetically. Acid halide substituents attached to rings are named using the suffix - carbonyl. 1 Gentilucci, Carboxylic Acid Derivatives Anhydrides 1. Symmetrical: replace the ending "acid" with "anhydride ". 2. Asymmetrical: select the longest continuous carbon chain, containing the carboxylic acid group, and derive the parent name by replacing the -e ending with -oic anhydride . 3. Number the carbon chain, beginning at the end nearest to the acyl group. 4. Number the substituents and write the name, listing substituents alphabetically. Gentilucci, Carboxylic Acid Derivatives Amides are named by replacing the ending -oic acid with -amide . 1. Select the longest continuous carbon chain, containing the acyl group, and derive the parent name by replacing the -e ending with -amide . 2. Number the carbon chain, beginning at the end nearest to the acyl group. 3. Number the substituents and write the name, listing substituents alphabetically. 4. If the nitrogen atom is further substituted, the substituents are preceded by N- to indicate that they are attached to the nitrogen. Acid halide substituents attached to rings are named using the suffix - carboxamide. 2 Gentilucci, Carboxylic Acid Derivatives Carboxylate esters 1. Select the longest continuous carbon chain containing the acyl group, and derive the parent name by replacing the -e ending with –oate . -

Indium Promoted-Convenient Method for Acylation of Alc이iols with Acyl Chlorides
Communications to the Editor Bull. Korean Chem. Soc. 2003, Vol. 24, No. 2 155 Indium Promoted-Convenient Method for Acylation of Alc이iols with Acyl Chlorides Dae Hyan Cho, Joong Gon Kim,f and Doo Ok Jang* Department of Chemistry, Yonsei University, Wonju 220-710, Korea ‘Biotechnology Division, Hanwha Chemical R & D Center, Daejeon 305-345, Korea Received November 9, 2002 Key Words : Indium, Alcohol, Acylation, Acyl chloride Even though various reagents for coupling of alcohols tions for the acylation of alcohols with acyl chlorides in the with carboxylic acids and transesterification of esters have presence of indium. The results are summarized in Table 1. been developed,1 there is still a great demand for a process Reaction of 2 (1 equiv) with 1 (1 equiv) in the presence of by using acyl chlorides for the acylation of alcohols in the indium (1 equiv) in CH3CN at room temperature produced case of substrates having steric hindrance or low reactivity. the corresponding ester in only 21% yield and the starting The acylation of alcohols with acyl chlorides is commonly acyl chloride and alcohol were recovered. The optimal yield carried out in the presence of tertiary amines such as 4- of the ester was attained with 3 equiv of 1 or 2 in the presence (methylamino) pyridine or 4 -pyrrolidinopyridine.2 Many of 3 equiv of indium. The solvent effect of acylation of 2 methods for the acylation of alcohols with acyl chlorides with 1 in the presence of indium was studied. The reaction have been developed using a variety of reagents.3 Most proceeded efficiently in common organic solvents such as recently, benzoylation of alcohols with lithium perchlorate DMF, Et2。,THF or CHzCh whereas non-polar solvents hase been reported.4 However, these methods have their own such as n-hexane or benzene gave poor yields of the ester. -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

Derivatives of Carboxylic Acids
Acylation A4 1 DERIVATIVES OF CARBOXYLIC ACIDS ACYL (ACID) CHLORIDES - RCOCl ACID ANHYDRIDES - (RCO)2O named from corresponding acid named from corresponding acid remove -ic add -yl chloride remove acid add anhydride CH3COCl ethanoyl chloride (CH3CO)2O ethanoic anhydride C6H5COCl benzene carbonyl chloride δ− δ+ O δ− CH C O 3 δ− δ+ O δ+ CH3 C δ− CH3 C δ− Cl O bonding in acyl chlorides bonding in acid anhydrides Chemical Properties • colourless liquids which fume in moist air • acyl chlorides are more reactive than anhydrides • attacked at the positive carbon centre by nucleophiles • nucleophiles include water, alcohols, ammonia and amines • undergo addition-elimination reactions Uses of Acylation Industrially Manufacture of Cellulose acetate - making fibres Aspirin (acetyl salicylic acid) - analgaesic Ethanoic anhydride is more useful • cheaper • less corrosive • less vulnerable to hydrolysis • less dangerous reaction Laboratory Used to make carboxylic acid, esters, amines, N-substituted amines Ethanoyl chloride is used as it • is more reactive • gives a cleaner reaction Q.1 Investigate how aspirin is made industrially and in the laboratory. Why are the reagents and chemicals different? What properties of Aspirin make it such a useful drug? 2 A4 Acylation ADDITION ELIMINATION REACTIONS - OVERVIEW Mechanism • species attacked by nucleophiles at the positive carbon end of the C=O bond • the nucleophile adds to the molecule • either Cl or RCOO¯ is eliminated • a proton is removed General example - with water ACID CHLORIDES H Cl + Cl H O O -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Revision Guide
Revision Guide Chemistry - Unit 3 Physical and Inorganic Chemistry GCE A Level WJEC These notes have been authored by experienced teachers and are provided as support to students revising for their GCE A level exams. Though the resources are comprehensive, they may not cover every aspect of the specification and do not represent the depth of knowledge required for each unit of work. 1 Content Page Section 2 3.1 – Redox and standard electrode potential 13 3.2 - Redox reactions 20 3.3 - Chemistry of the p-block 30 3.4 - Chemistry of the d-block transition metals 35 3.5 - Chemical kinetics 44 3.6 - Enthalpy changes for solids and solutions 50 3.7 - Entropy and feasibility of reactions 53 3.8 - Equilibrium constants 57 3.9 - Acid-base equilibria 66 Acknowledgements 2 3.1 – Redox and standard electrode potential Redox reactions In AS, we saw that in redox reactions, something is oxidised and something else is reduced (remember OILRIG – this deals with loss and gain of electrons). Another way that we can determine if a redox reaction has happened is by using oxidation states or numbers (see AS revision guide pages 2 and 44). You need to know that: - • oxidation is loss of electrons • reduction is gain of electrons • an oxidising agent is a species that accepts electrons, thereby helping oxidation. It becomes reduced itself in the process. • a reducing agent is a species that donates electrons, thereby helping reduction. It becomes oxidised itself in the process. You also should remember these rules for assigning oxidation numbers in a compound: - 1 All elements have an oxidation number of zero (including diatomic molecules like H2) 2 Hydrogen is 1 unless it’s with a Group 1 metal, then it’s -1 3 Oxygen is -2 (unless it’s a peroxide when it’s -1, or reacted with fluorine, when it’s +2). -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

N Goalby Chemrevise.Org 1 Reaction with Water
Carboxylic acid derivatives: Acyl Chlorides and Acid Anhydrides Acyl Chlorides Acid Anhydrides Acid anhydrides have a similar reactivity to O Acyl chlorides are much O acyl chlorides and therefore bring about the CH3 C more reactive than same changes in functional groups. carboxylic acids CH3 C Cl O ethanoyl chloride The main difference is the by-products. CH3 C Acyl chlorides mostly give off HCl. Acid O anhydrides give off RCOOH ethanoic anhydride. Explaining reactivity Many of the reactions of the carboxylic acid derivatives follow the pattern below with an attack by an nucleophile. O O CH3 C + :X- CH3 C + :W- On a simplistic level, the stronger the electron attracting power of ‘W’, the W X more positive the carbon, and the more attractive the carbon is to Where –W: and :W- can be –Cl and Cl- (acyl chlorides) nucleophiles. - or -OCH3 and OCH3 (esters) The relative attractive powers of the –W: are - `or -NH2 and NH2 (amides) -Cl > -OH > -OCH3 > -NH2 Therefore in the case of hydrolysis reactions, acyl chlorides are highly reactive and will be hydrolysed by weak nucleophiles such as water. Amides and Esters contain only weak electron attracting W groups and need strong nucleophiles such as hydroxide ions in NaOH to hydrolyse. This difference in reactivity is caused by a combination of two factors 1. the electronegativity of the Cl’s, N’s and O’s causing electron density to be withdrawn from the carbon making the carbon more positive and more attractive to nucleophiles- This factor makes them more reactive. 2. delocalisation of the lone pairs on these atoms into the carbonyl system which reduces the reactivity. -
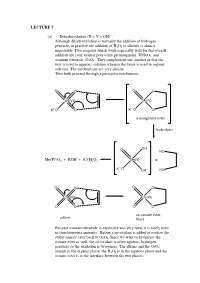
A Manganate Ester Hydrolysis Mn(IV)
LECTURE 7 (a) Dihydroxylation (X = Y = OH) Although dihydroxylation is formally the addition of hydrogen peroxide, in practice the addition of H2O2 to alkenes is almost impossible. Two reagents which work especially well for this overall addition are cold, neutral potassium permanganate, KMnO4, and osmium tetroxide, OsO4. They complement one another in that the first is used in aqueous solution whereas the latter is used in organic solvents. The mechanisms are very similar. Thus both proceed through a pericyclic mechanism: O O O O Mn(VII) Mn(V) +- K+-O O K O O a manganate ester hydrolysis O OH HO Mn(IV)O2 + KOH + 0.5 H2O2 Mn(V) + +- K O OH HO O O O O Os(VIII) Os(VI) O O O O an osmate ester, yellow black Because osmium tetroxide is expensive and very toxic it is rarely used in stoichiometric amounts. Rather a co-oxidant is added to oxidise the stable osmate ester back to OsO4. Since we want to hydrolyse the osmate ester as well, the co-oxidant is often aqueous hydrogen peroxide so the oxidation is two-phase. The alkene and the OsO4 remain in the organic phase, the H2O2 is in the aqueous phase and the osmate ester is at the interface between the two phases: H OH H OH H OsO4, + aq.H2O2, CH Cl H 2 2 OH H H OH (c) Epoxidation (X = Y = O) The reaction of alkenes with peroxy acids (RCO3H) leads to a cyclic ether, known as an epoxide, as follows: R R O O O H O H O O Although the reaction will work with peroxyacetic acid (R = Me) it works best with peroxy acids bearing electron-withdrawing groups e.g. -

Chapter 17 Aromatic Reactions
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step 1: Attack on the electrophile forms the sigma complex. Step 2: Loss of a proton gives the substitution product. => Chapter 17: Aromatics 2-Reactions Slide 17-3 Bromination of Benzene • Requires a stronger electrophile than Br2. • Use a strong Lewis acid catalyst, FeBr3. Br Br + - FeBr3 Br Br FeBr3 H H H H H H + - Br _ Br Br FeBr3 + + FeBr4 H H H H H H Br + HBr => Chapter 17: Aromatics 2-Reactions Slide 17-4 2 Comparison with Alkenes • Cyclohexene adds Br2, ΔH = -121 kJ • Addition to benzene is endothermic, not normally seen. • Substitution of Br for H retains aromaticity, ΔH = -45 kJ. • Formation of sigma complex is rate-limiting. => Chapter 17: Aromatics 2-Reactions Slide 17-5 Energy Diagram for Bromination => Chapter 17: Aromatics 2-Reactions Slide 17-6 3 Chlorination and Iodination • Chlorination is similar to bromination. Use AlCl3 as the Lewis acid catalyst. • Iodination requires an acidic oxidizing agent, like nitric acid, which oxidizes the iodine to an iodonium ion. + + H + HNO3 + 1/2 I2 I + NO2 + H2O => Chapter 17: Aromatics 2-Reactions Slide 17-7 Nitration of Benzene Use sulfuric acid with nitric acid to form the nitronium ion electrophile. + NO2 then forms a sigma complex with benzene, loses H+ to form nitrobenzene. Chapter 17: Aromatics 2-Reactions Slide 17-8 4 Sulfonation Sulfur trioxide, SO3, in fuming sulfuric acid is the electrophile. _ O O O O S S + S + S + _ _ O O O O O O O O Chapter 17: Aromatics 2-Reactions Slide 17-9 Desulfonation • All steps are reversible, so sulfonic acid group can be removed by heating in dilute sulfuric acid. -

The Anodic Oxidation of Maleic Acid
Scholars' Mine Masters Theses Student Theses and Dissertations 1966 The anodic oxidation of maleic acid Larry D. Gilmartin Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Chemical Engineering Commons Department: Recommended Citation Gilmartin, Larry D., "The anodic oxidation of maleic acid" (1966). Masters Theses. 5776. https://scholarsmine.mst.edu/masters_theses/5776 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. THE ANODIC OXIDATION OF MALEIC ACID BY LARRY D. GILMARTIN A THESIS submitted to the faculty of THE UNIVERSITY OF MISSOURI AT ROLLA in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE IN CHEMICAL ENGINEERING Rolla, Missouri 1966 Approved by (advisor) THE ANODIC OXIDATION OF MALEIC ACID Larry D. Gilmartin ABSTRACT The purpose of this investigation was to determine the mechanism of the anodic oxidation of maleic acid on platinized-platinum electrodes at 80°C. Current density-potential studies were conducted varying the parameters of maleic acid concentration and pH. The faradaic efficiency of the oxidation of maleic acid to yield co 2 was determined. The effect of temperature on current density was also studied to determine the activation energy for the reaction. The oxidation of maleic acid occurred only in acidic solutions. The faradaic efficiency was found to be ap proximately 97 ± 5 per cent. A linear Tafel region was found which had a slope of 145 - 170 millivolts <~ 2.3RT/aF). -

Approach and Synthesis of Strychnos Alkaloids
N° d'ordre : 4155 THÈSE Présentée à L'UNIVERSITÉ BORDEAUX I ÉCOLE DOCTORALE DES SCIENCES CHIMIQUES par Dawood Hosni DAWOOD POUR OBTENIR LE GRADE DE DOCTEUR SPÉCIALITÉ : CHIMIE ORGANIQUE ********************* TOWARDS THE SYNTHESIS OF MONOTERPENOIDS INDOLE ALKALOIDS OF THE ASPIDOSPERMATAN AND STRYCHNAN TYPE ********************* Soutenue le: 17 décembre 2010 Après avis de: MM. PIVA Olivier Professeur, Claude Bernard Lyon 1 Rapporteur PALE Patrick Professeur, Louis Pasteur Strasbourg 1 Rapporteur Devant la commission d'examen formée de : MM. PIVA Olivier Professeur, Claude Bernard Lyon 1 Rapporteur PALE Patrick Professeur, Louis Pasteur Strasbourg 1 Rapporteur POISSON Jean-François Chargé de recherche, CNRS Examinateur VINCENT Jean-Marc Directeur de recherche, CNRS Examinateur LANDAIS Yannick Professeur, Bordeaux 1 Directeur de thèse ROBERT Frédéric Chargé de recherche, CNRS Codirecteur de thèse - 2010 - Abbreviations ∆: reflux °C: celsius degrees Ac: acetyle ALB Aluminium Lithium bis(binaphthoxide) complex AIBN : azobis(isobutyronitrile) aq.: aqueous Ar : aromatic BINAP : 2,2'-bis(diphenylphosphino)-1,1'-binaphthyle BINAPO : 2-diphenylphosphino-2'-diphenylphosphinyl-1,1'-binaphthalene BINOL: 1,1’-bi-2-naphthol Boc: tert-butyloxycarbonyle BOX: Bisoxazoline Bz : benzoyle Bn: benzyle cat. : catalytic DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene DCM: dichloromethane DCC: dicyclohexacarbodiimide dr.: diastereomeric ratio DIBAL-H: diisobutylaluminium hydride DIPEA: diisopropyléthylamine (Hünig Base) DMAP: dimethylaminopyridine DME: dimethoxyethane