Oxidative Kinetic Study of Fluoroquinolone Pharmaceuticals in Acidic/Basic Aqueous Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

A Review of Enrofloxacin for Veterinary Use Tessa Trouchon, Sebastien Lefebvre
A Review of Enrofloxacin for Veterinary Use Tessa Trouchon, Sebastien Lefebvre To cite this version: Tessa Trouchon, Sebastien Lefebvre. A Review of Enrofloxacin for Veterinary Use. Open Journal of Veterinary Medicine, 2016, 6 (2), pp.40-58. 10.4236/ojvm.2016.62006. hal-01503397 HAL Id: hal-01503397 https://hal.archives-ouvertes.fr/hal-01503397 Submitted on 7 Apr 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NoDerivatives| 4.0 International License Open Journal of Veterinary Medicine, 2016, 6, 40-58 Published Online February 2016 in SciRes. http://www.scirp.org/journal/ojvm http://dx.doi.org/10.4236/ojvm.2016.62006 A Review of Enrofloxacin for Veterinary Use Tessa Trouchon, Sébastien Lefebvre USC 1233 INRA-Vetagro Sup, Veterinary School of Lyon, Marcy l’Etoile, France Received 12 January 2016; accepted 21 February 2016; published 26 February 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract This review outlines the current knowledge on the use of enrofloxacin in veterinary medicine from biochemical mechanisms to the use in the field conditions and even resistance and ecotoxic- ity. -

An Investigation of Class 1 Integrons and Integrase Gene In
AN INVESTIGATION OF CLASS 1 INTEGRONS AND INTEGRASE GENE IN ESCHERICHIA COLI AND MANNHEIMIA HAEMOLYTICA FROM BEEF CATTLE IN WESTERN CANADA A Thesis Presented to The Faculty of Graduate Studies of The University of Guelph by MATTHEW LESLIE In partial fulfillment of requirements for the degree of Master of Science May, 2011 © Matthew Leslie, 2011 Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington OttawaONK1A0N4 Ottawa ON K1A 0N4 Canada Canada Your file Vote reference ISBN: 978-0-494-82791-8 Our We Notre r6f6rence ISBN: 978-0-494-82791-8 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distribute and sell theses monde, a des fins commerciaies ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

AMEG Categorisation of Antibiotics
12 December 2019 EMA/CVMP/CHMP/682198/2017 Committee for Medicinal Products for Veterinary use (CVMP) Committee for Medicinal Products for Human Use (CHMP) Categorisation of antibiotics in the European Union Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 29 October 2018 Adopted by the CVMP for release for consultation 24 January 2019 Adopted by the CHMP for release for consultation 31 January 2019 Start of public consultation 5 February 2019 End of consultation (deadline for comments) 30 April 2019 Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 19 November 2019 Adopted by the CVMP 5 December 2019 Adopted by the CHMP 12 December 2019 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Categorisation of antibiotics in the European Union Table of Contents 1. Summary assessment and recommendations .......................................... 3 2. Introduction ............................................................................................ 7 2.1. Background ........................................................................................................ -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Revision Guide
Revision Guide Chemistry - Unit 3 Physical and Inorganic Chemistry GCE A Level WJEC These notes have been authored by experienced teachers and are provided as support to students revising for their GCE A level exams. Though the resources are comprehensive, they may not cover every aspect of the specification and do not represent the depth of knowledge required for each unit of work. 1 Content Page Section 2 3.1 – Redox and standard electrode potential 13 3.2 - Redox reactions 20 3.3 - Chemistry of the p-block 30 3.4 - Chemistry of the d-block transition metals 35 3.5 - Chemical kinetics 44 3.6 - Enthalpy changes for solids and solutions 50 3.7 - Entropy and feasibility of reactions 53 3.8 - Equilibrium constants 57 3.9 - Acid-base equilibria 66 Acknowledgements 2 3.1 – Redox and standard electrode potential Redox reactions In AS, we saw that in redox reactions, something is oxidised and something else is reduced (remember OILRIG – this deals with loss and gain of electrons). Another way that we can determine if a redox reaction has happened is by using oxidation states or numbers (see AS revision guide pages 2 and 44). You need to know that: - • oxidation is loss of electrons • reduction is gain of electrons • an oxidising agent is a species that accepts electrons, thereby helping oxidation. It becomes reduced itself in the process. • a reducing agent is a species that donates electrons, thereby helping reduction. It becomes oxidised itself in the process. You also should remember these rules for assigning oxidation numbers in a compound: - 1 All elements have an oxidation number of zero (including diatomic molecules like H2) 2 Hydrogen is 1 unless it’s with a Group 1 metal, then it’s -1 3 Oxygen is -2 (unless it’s a peroxide when it’s -1, or reacted with fluorine, when it’s +2). -

Belgian Veterinary Surveillance of Antibacterial Consumption National
Belgian Veterinary Surveillance of Antibacterial Consumption National consumption report 2020 Publication : 22 June 2021 1 SUMMARY This annual BelVet-SAC report is now published for the 12th time and describes the antimicrobial use (AMU) in animals in Belgium in 2020 and the evolution since 2011. For the third year this report combines sales data (collected at the level of the wholesalers-distributors and the compound feed producers) and usage data (collected at farm level). This allows to dig deeper into AMU at species and farm level in Belgium. With a consumption of 87,6 mg antibacterial compounds/kg biomass an increase of +0.2% is seen in 2020 in comparison to 2019. The increase seen in 2020 is spread over both pharmaceuticals (+0.2%) and antibacterial premixes (+4.0%). This unfortunately marks the end of a successful reduction in antibacterial product sales that was seen over the last 6 years resulting in a cumulative reduction of -40,2% since 2011. The gap seen in the coverage of the sales data with the Sanitel-Med collected usage data increased substantially compared to 2019, meaning continuous efforts need to be taken to ensure completeness of the collected usage data. When looking at the evolution in the number of treatment days (BD100) at the species level, as calculated from the SANITEL- MED use data, use increased in poultry (+5,0%) and veal calves (+1,9%), while it decreased in pigs (-3,1%). However, the numerator data for this indicator remain to be updated for 2020, potentially influencing the reliability of the result. -

DANMAP 2016 - Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark
Downloaded from orbit.dtu.dk on: Oct 09, 2021 DANMAP 2016 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark Borck Høg, Birgitte; Korsgaard, Helle Bisgaard; Wolff Sönksen, Ute; Bager, Flemming; Bortolaia, Valeria; Ellis-Iversen, Johanne; Hendriksen, Rene S.; Borck Høg, Birgitte; Jensen, Lars Bogø; Korsgaard, Helle Bisgaard Total number of authors: 27 Publication date: 2017 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Borck Høg, B. (Ed.), Korsgaard, H. B. (Ed.), Wolff Sönksen, U. (Ed.), Bager, F., Bortolaia, V., Ellis-Iversen, J., Hendriksen, R. S., Borck Høg, B., Jensen, L. B., Korsgaard, H. B., Pedersen, K., Dalby, T., Træholt Franck, K., Hammerum, A. M., Hasman, H., Hoffmann, S., Gaardbo Kuhn, K., Rhod Larsen, A., Larsen, J., ... Vorobieva, V. (2017). DANMAP 2016 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Statens Serum Institut, National Veterinary Institute, Technical University of Denmark National Food Institute, Technical University of Denmark. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

NVA237 / Glycopyrronium Bromide Multinational, Multi-Database Drug
Quantitative Safety & Epidemiology NVA237 / Glycopyrronium bromide Non-interventional Final Study Report NVA237A2401T Multinational, multi-database drug utilization study of inhaled NVA237 in Europe Author Document Status Final Date of final version 28 April 2016 of the study report EU PAS register ENCEPP/SDPP/4845 number Property of Novartis Confidential May not be used, divulged, published or otherwise disclosed without the consent of Novartis NIS Report Template Version 2.0 August-13-2014 Novartis Confidential Page 2 Non-interventional study report NVA237A/Seebri® Breezhaler®/CNVA237A2401T PASS information Title Multinational, multi-database drug utilization study of inhaled NVA237 in Europe –Final Study Report Version identifier of the Version 1.0 final study report Date of last version of 28 April 2016 the final study report EU PAS register number ENCEPP/SDPP/4845 Active substance Glycopyrronium bromide (R03BB06) Medicinal product Seebri®Breezhaler® / Tovanor®Breezhaler® / Enurev®Breezhaler® Product reference NVA237 Procedure number SeebriBreezhaler: EMEA/H/C/0002430 TovanorBreezhaler: EMEA/H/C/0002690 EnurevBreezhaler: EMEA/H/C0002691 Marketing authorization Novartis Europharm Ltd holder Frimley Business Park Camberley GU16 7SR United Kingdom Joint PASS No Research question and In the context of the NVA237 marketing authorization objectives application, the Committee for Medicinal Products for Human Use (CHMP) recommended conditions for marketing authorization and product information and suggested to conduct a post-authorization -
A Manganate Ester Hydrolysis Mn(IV)
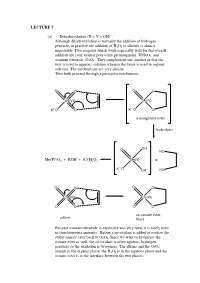
LECTURE 7 (a) Dihydroxylation (X = Y = OH) Although dihydroxylation is formally the addition of hydrogen peroxide, in practice the addition of H2O2 to alkenes is almost impossible. Two reagents which work especially well for this overall addition are cold, neutral potassium permanganate, KMnO4, and osmium tetroxide, OsO4. They complement one another in that the first is used in aqueous solution whereas the latter is used in organic solvents. The mechanisms are very similar. Thus both proceed through a pericyclic mechanism: O O O O Mn(VII) Mn(V) +- K+-O O K O O a manganate ester hydrolysis O OH HO Mn(IV)O2 + KOH + 0.5 H2O2 Mn(V) + +- K O OH HO O O O O Os(VIII) Os(VI) O O O O an osmate ester, yellow black Because osmium tetroxide is expensive and very toxic it is rarely used in stoichiometric amounts. Rather a co-oxidant is added to oxidise the stable osmate ester back to OsO4. Since we want to hydrolyse the osmate ester as well, the co-oxidant is often aqueous hydrogen peroxide so the oxidation is two-phase. The alkene and the OsO4 remain in the organic phase, the H2O2 is in the aqueous phase and the osmate ester is at the interface between the two phases: H OH H OH H OsO4, + aq.H2O2, CH Cl H 2 2 OH H H OH (c) Epoxidation (X = Y = O) The reaction of alkenes with peroxy acids (RCO3H) leads to a cyclic ether, known as an epoxide, as follows: R R O O O H O H O O Although the reaction will work with peroxyacetic acid (R = Me) it works best with peroxy acids bearing electron-withdrawing groups e.g. -

Pharmacology for Veterinary Medicine Students Part II by Staff Members Depatment of Pharmacology Faculty of Veterinary Medicine
Pharmacology for veterinary medicine students Part II By Staff members Depatment of Pharmacology Faculty of Veterinary Medicine Beni-Suef University 2019-2020 Item Page Drugs affecting etabolism……………………………………………….3 Antibiotics……………………………………………………………...17 Sulphonamides and Other antimicrobials……………………………….47 Antifungal drugs………………………………………………………...62 Antitubercular drugs……………………………………….………....…66 Antiparasitics………………………………………………..………….68 Antiprotozoals…………………………………………….……………78 Antiseptics and disinfectants……………………………….…….…….85 Antiviral agents…………………………………………….………..…90 Cancer chemotherapy………………………………………..………….97 Drug affecting endocrine system………………………………………101 Clinical pharmacology………………………………………….……. 116 Drug toxicology……………………………………………………… 125 Fish pharmacology…………………………………………………… 137 2 Fluids, electrolytes and acid-base therapy About 60 % of a normal animal's body weight is composed of water, fat, age, obese and older animals tend to have a small percentage of water of this 60 %, 33 % is within the body cells and is referred to as intracellular fluid compartment (ICF). The remaining 27 % is outside (between) the cells and is referred to as the extracellular fluid compartment (ECF). ECF may be further divided into sub-compartments (plasma 5 %, interstitial fluid 8 %, transcellular fluid 2 %, dense connective tissue and bone 12 %). Sodium is principally responsible for ECF osmolality. Osmolality in the ICF is principally determined by potassium, magnesium, phosphates and proteins. Na-K ATPase that is present in most cellular membranes (except for most canine and feline red blood cell membranes) maintains a very low intracellular Na concentration. Hence, if Na is added to the ECF it stays there and increases the osmolality and tonicity consequently, water is be drawn out of the ICF and into the ECF compartments until the osmolalities of the two fluid compartments are nearly equal. Chloride atoms usually follow Na atoms in order to maintain electroneurality. -

Effects of Chlortetracycline and Copper Supplementation on Levels of Antimicrobial Resistance in the Feces of Weaned Pigs
EFFECTS OF CHLORTETRACYCLINE AND COPPER SUPPLEMENTATION ON LEVELS OF ANTIMICROBIAL RESISTANCE IN THE FECES OF WEANED PIGS by GETAHUN EJETA AGGA DVM, Addis Ababa University, 2003 MSc, Utrecht University, 2008 AN ABSTRACT OF A DISSERTATION submitted in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Department of Diagnostic Medicine/Pathobiology College of Veterinary Medicine KANSAS STATE UNIVERSITY Manhattan, Kansas 2013 Abstract The use of antibiotics in food animals is of major concern as a purported cause of antimicrobial resistance (AMR) in human pathogens; as a result, alternatives to in-feed antibiotics such as heavy metals have been proposed. The effect of copper and CTC supplementation in weaned pigs on AMR in the gut microbiota was evaluated. Four treatment groups: control, copper, chlortetracycline (CTC), and copper plus CTC were randomly allocated to 32 pens with five pigs per pen. Fecal samples (n = 576) were collected weekly from three pigs per pen over six weeks and two Escherichia coli isolates per sample were tested phenotypically for antimicrobial and copper susceptibilities and genotypically for the presence of tetracycline (tet), copper (pcoD) and ceftiofur (blaCMY-2) resistance genes. CTC-supplementation significantly increased tetracycline resistance and susceptibility to copper when compared with the control group. Copper supplementation decreased resistance to most of the antibiotics, including cephalosporins, over all treatment periods. However, copper supplementation did not affect minimum inhibitory concentrations of copper or detection of pcoD. While tetA and blaCMY-2 genes were associated with a higher multi-drug resistance (MDR), tetB and pcoD were associated with lower MDR. Supplementations of CTC or copper alone were associated with increased tetB prevalence; however, their combination was paradoxically associated with reduced prevalence.