The Impact of Potassium Manganate (Vii)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

Exactly As Received Mic 61-929 MERRYMAN, Earl L Ew Is. THE
This dissertation has been microfilmed exactly as received Mic 61-929 MERRYMAN, Earl Lewis. THE ISOTOPIC EXCHANGE REACTION BETWEEN Mn AND MnO” . 4 The Ohio State University, Ph.D, 1960 Chemistry, physical University Microfilms, Inc., Ann Arbor, Michigan THE ISOTOPIC EXCHANGE REACTION BETTAIEEN Mn** AND ItaO ^ DISSERTATION Presented in P&rtial Fulfillment of the Requirements for the Degree Doctor of Philosophy In the Graduate School of The Ohio S tate U niversity By Earl Lewis Ferryman, B.Sc* The Ohio State University I960 Approved by Department oy Chenletry 1C mnriEDGiBiT The author wlshea to e:qpr«as his approoiation to Profoaaor Alfred B. Garrett for hie superrieion and enocur- agement during the oouree of this research* and for his sincere interest in mj eelfare both as an undergraduate and graduate student at Ohio State University. I also wish to thank the Ohio State University Cheidstry Depsurtnent for the Assistant ships granted me during the 1 9 5 6* 7 "^ aeademlo years. The author also gratefully acknowledges the Fellowships granted me by the American Cyansuald Company during the 1959*60 academic year and by the National Science Foundation during the Summer Q u a rte r of I960* i i TABI£ OP CONTEHTS PAOE INTRODUCTION ............................................................................................................... 1 Àpplloationa of Radloaotirlty in Chomiatry 1 The Problem and Its H latory ....................................................... .. 1 The Problem Reeulting from Early Work 5 Statement of the Problem .......................... -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Revision Guide
Revision Guide Chemistry - Unit 3 Physical and Inorganic Chemistry GCE A Level WJEC These notes have been authored by experienced teachers and are provided as support to students revising for their GCE A level exams. Though the resources are comprehensive, they may not cover every aspect of the specification and do not represent the depth of knowledge required for each unit of work. 1 Content Page Section 2 3.1 – Redox and standard electrode potential 13 3.2 - Redox reactions 20 3.3 - Chemistry of the p-block 30 3.4 - Chemistry of the d-block transition metals 35 3.5 - Chemical kinetics 44 3.6 - Enthalpy changes for solids and solutions 50 3.7 - Entropy and feasibility of reactions 53 3.8 - Equilibrium constants 57 3.9 - Acid-base equilibria 66 Acknowledgements 2 3.1 – Redox and standard electrode potential Redox reactions In AS, we saw that in redox reactions, something is oxidised and something else is reduced (remember OILRIG – this deals with loss and gain of electrons). Another way that we can determine if a redox reaction has happened is by using oxidation states or numbers (see AS revision guide pages 2 and 44). You need to know that: - • oxidation is loss of electrons • reduction is gain of electrons • an oxidising agent is a species that accepts electrons, thereby helping oxidation. It becomes reduced itself in the process. • a reducing agent is a species that donates electrons, thereby helping reduction. It becomes oxidised itself in the process. You also should remember these rules for assigning oxidation numbers in a compound: - 1 All elements have an oxidation number of zero (including diatomic molecules like H2) 2 Hydrogen is 1 unless it’s with a Group 1 metal, then it’s -1 3 Oxygen is -2 (unless it’s a peroxide when it’s -1, or reacted with fluorine, when it’s +2). -

Justification for the Selection of a Corap Substance
JUSTIFICATION DOCUMENT FOR THE SELECTION OF A CORAP SUBSTANCE _________________________________________________________________ Justification Document for the Selection of a CoRAP Substance Substance Name (public name): Potassium permanganate EC Number: 231-760-3 CAS Number: 7722-64-7 Authority: France Date: 21/03/2017 Cover Note This document has been prepared by the evaluating Member State given in the CoRAP update. JUSTIFICATION DOCUMENT FOR THE SELECTION OF A CORAP SUBSTANCE _______________________________________________________________ Table of Contents 1 IDENTITY OF THE SUBSTANCE 3 1.1 Other identifiers of the substance 3 1.2 Similar substances/grouping possibilities 4 2 OVERVIEW OF OTHER PROCESSES / EU LEGISLATION 4 3 HAZARD INFORMATION (INCLUDING CLASSIFICATION) 5 3.1 Classification 5 3.1.1 Harmonised Classification in Annex VI of the CLP 5 3.1.2 Self classification 5 3.1.3 Proposal for Harmonised Classification in Annex VI of the CLP 5 4 INFORMATION ON (AGGREGATED) TONNAGE AND USES 6 4.1 Tonnage and registration status 6 4.2 Overview of uses 6 5. JUSTIFICATION FOR THE SELECTION OF THE CANDIDATE CORAP SUBSTANCE 8 5.1. Legal basis for the proposal 8 5.2. Selection criteria met (why the substance qualifies for being in CoRAP) 8 5.3. Initial grounds for concern to be clarified under Substance Evaluation 8 5.4. Preliminary indication of information that may need to be requested to clarify the concern 9 5.5. Potential follow-up and link to risk management 10 EC no 231-760-3 MSCA - France Page 2 of 10 JUSTIFICATION DOCUMENT FOR THE -

Safe Handling and Disposal of Chemicals Used in the Illicit Manufacture of Drugs
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Safe handling and disposal of chemicals used in the illicit manufacture of drugs United Nations publication USD 26 Printed in Austria ISBN 978-92-1-148266-9 Sales No. E.11.XI.14 ST/NAR/36/Rev.1 V.11-83777—September*1183777* 2011—300 Guidelines for the Safe handling and disposal of chemicals used in the illlicit manufacture of drugs UNITED NATIONS New York, 2011 Symbols of United Nations documents are composed of letters combined with figures. Mention of such symbols indicates a reference to a United Nations document. ST/NAR/36/Rev.1 UNITED NATIONS PUBLICATION Sales No. E.11.XI.14 ISBN 978-92-1-148266-9 eISBN 978-92-1-055160-1 © United Nations, September 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Requests for permission to reproduce this work are welcomed and should be sent to the Secretary of the Publications Board, United Nations Headquarters, New York, N.Y. 10017, U.S.A. or also see the website of the Board: https://unp.un.org/Rights.aspx. Governments and their institutions may reproduce this work without prior authoriza- tion but are requested to mention the source and inform the United Nations of such reproduction. -
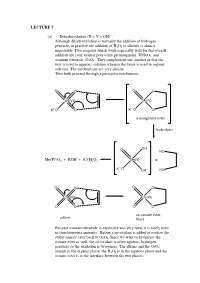
A Manganate Ester Hydrolysis Mn(IV)
LECTURE 7 (a) Dihydroxylation (X = Y = OH) Although dihydroxylation is formally the addition of hydrogen peroxide, in practice the addition of H2O2 to alkenes is almost impossible. Two reagents which work especially well for this overall addition are cold, neutral potassium permanganate, KMnO4, and osmium tetroxide, OsO4. They complement one another in that the first is used in aqueous solution whereas the latter is used in organic solvents. The mechanisms are very similar. Thus both proceed through a pericyclic mechanism: O O O O Mn(VII) Mn(V) +- K+-O O K O O a manganate ester hydrolysis O OH HO Mn(IV)O2 + KOH + 0.5 H2O2 Mn(V) + +- K O OH HO O O O O Os(VIII) Os(VI) O O O O an osmate ester, yellow black Because osmium tetroxide is expensive and very toxic it is rarely used in stoichiometric amounts. Rather a co-oxidant is added to oxidise the stable osmate ester back to OsO4. Since we want to hydrolyse the osmate ester as well, the co-oxidant is often aqueous hydrogen peroxide so the oxidation is two-phase. The alkene and the OsO4 remain in the organic phase, the H2O2 is in the aqueous phase and the osmate ester is at the interface between the two phases: H OH H OH H OsO4, + aq.H2O2, CH Cl H 2 2 OH H H OH (c) Epoxidation (X = Y = O) The reaction of alkenes with peroxy acids (RCO3H) leads to a cyclic ether, known as an epoxide, as follows: R R O O O H O H O O Although the reaction will work with peroxyacetic acid (R = Me) it works best with peroxy acids bearing electron-withdrawing groups e.g. -

Chapter-17 Antimicrobials
CHAPTER-17 ANTIMICROBIALS Hydrogen peroxide Hydrogen peroxide (H2O2) is the simplest peroxide (a compound with an oxygen-oxygen single bond). It is also a strong oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. Due to its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent. The oxidizing capacity of hydrogen peroxide is so strong that it is considered a highly reactive oxygen species. Hydrogen peroxide is therefore used as a propellant in rocketry. Organisms also naturally produce hydrogen peroxide as a by-product of oxidative metabolism. Consequently, nearly all living things (specifically, all obligate and facultative aerobes) possess enzymes known as catalase peroxidases, which harmlessly and catalytically decompose low concentrations of hydrogen peroxide. Reactions Manganese dioxide decomposing a very dilute solution of hydrogen peroxide Hydrogen peroxide decomposes (disproportionates) exothermically into water and oxygen gas spontaneously: 2 H2O2 → 2 H2O + O2 Redox reactions In acidic solutions, H2O2 is one of the most powerful oxidizers known—stronger than chlorine, chlorine dioxide, and potassium permanganate. Also, through catalysis, H2O2 can be converted into hydroxyl radicals (•OH), which are highly reactive. Therapeutic use Hydrogen peroxide is generally recognized as safe (GRAS) as an antimicrobial agent, an oxidizing agent and for other purposes by the U.S. FDA. For example, 35% hydrogen peroxide is used to prevent infection transmission in the hospital environment, and hydrogen peroxide vapor is registered with the US EPA as a sporicidal sterilant. It is a common misconception that hydrogen peroxide is a disinfectant or antiseptic for treating wounds. -

The Anodic Oxidation of Maleic Acid
Scholars' Mine Masters Theses Student Theses and Dissertations 1966 The anodic oxidation of maleic acid Larry D. Gilmartin Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Chemical Engineering Commons Department: Recommended Citation Gilmartin, Larry D., "The anodic oxidation of maleic acid" (1966). Masters Theses. 5776. https://scholarsmine.mst.edu/masters_theses/5776 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. THE ANODIC OXIDATION OF MALEIC ACID BY LARRY D. GILMARTIN A THESIS submitted to the faculty of THE UNIVERSITY OF MISSOURI AT ROLLA in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE IN CHEMICAL ENGINEERING Rolla, Missouri 1966 Approved by (advisor) THE ANODIC OXIDATION OF MALEIC ACID Larry D. Gilmartin ABSTRACT The purpose of this investigation was to determine the mechanism of the anodic oxidation of maleic acid on platinized-platinum electrodes at 80°C. Current density-potential studies were conducted varying the parameters of maleic acid concentration and pH. The faradaic efficiency of the oxidation of maleic acid to yield co 2 was determined. The effect of temperature on current density was also studied to determine the activation energy for the reaction. The oxidation of maleic acid occurred only in acidic solutions. The faradaic efficiency was found to be ap proximately 97 ± 5 per cent. A linear Tafel region was found which had a slope of 145 - 170 millivolts <~ 2.3RT/aF). -

Complexes of Ferrous Iron with Tannic Acid Fy J
Complexes of Ferrous Iron With Tannic Acid fy J. D. HEM :HEMISTRY OF IRON IN NATURAL WATER GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1459-D IITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1960 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. CONTENTS Page Abstract. _________________________________________________________ 75 Acknowledgments. ________________________________________________ 75 Organic complexing agents________-______-__-__-__-______-____-___-- 75 Tannic acid_______________________________________________________ 77 Properties ____________________________________________________ 78 Dissociation._________________________________________________ 78 Reducing action_____--_-______________________________________ 79 Laboratory studies_______________________________________________ 79 Analytical procedures__________________________________________ 80 Chemical reactions in test solutions._____________________________ 81 No tannic acid____________________-_________________-_--__ 84 Five parts per million of tannic acid- ________________________ 84 Fifty parts per million of tannic acid_____-________-____------ 85 Five hundred parts per million of tanni c acid _________________ 86 Rate of oxidation and precipitation of iron______________________ 87 Stability constants for tannic acid complexes______________________ 88 Comparison of determined and estimated Eh______________________ -

The Sealing Step in Aluminum Anodizing: a Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance
coatings Review The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance Stanley Udochukwu Ofoegbu * ,Fábio A.O. Fernandes and António B. Pereira Centre for Mechanical Technology and Automation (TEMA), Department of Mechanical Engineering, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal; [email protected] (F.A.O.F.); [email protected] (A.B.P.) * Correspondence: [email protected] Received: 21 January 2020; Accepted: 27 February 2020; Published: 1 March 2020 Abstract: Increasing demands for environmental accountability and energy efficiency in industrial practice necessitates significant modification(s) of existing technologies and development of new ones to meet the stringent sustainability demands of the future. Generally, development of required new technologies and appropriate modifications of existing ones need to be premised on in-depth appreciation of existing technologies, their limitations, and desired ideal products or processes. In the light of these, published literature mostly in the past 30 years on the sealing process; the second highest energy consuming step in aluminum anodization and a step with significant environmental impacts has been critical reviewed in this systematic review. Emphasis have been placed on the need to reduce both the energy input in the anodization process and environmental implications. The implications of the nano-porous structure of the anodic oxide on mass transport and chemical reactivity of relevant species during the sealing process is highlighted with a focus on exploiting these peculiarities, in improving the quality of sealed products. In addition, perspective is provided on plausible approaches and important factors to be considered in developing sealing procedures that can minimize the energy input and environmental impact of the sealing step, and ensure a more sustainable aluminum anodization process/industry.