Sis 0043.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

Microbial Study on Corrosion
AN INVESTIGATION OF MICROBIAL DIVERSITY AND MICROBIOLOGICALLY INFLUENCED CORROSION IN AUTOMOTIVE FUEL ENVIRONMENTS by Charles H.D. Williamson IV A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Environmental Science and Engineering). Golden, Colorado Date ____________________________ Signed: ___________________________ _ Charles H.D. Williamson IV Signed: ____________________________ Dr. John R. Spear Thesis Advisor Golden, Colorado Date ____________________________ Signed: ____________________________ Dr. John McCray Professor and Director Department of Civil and Environmental Engineering ii ABSTRACT Microbial contamination of fuels can cause issues such as biofouling, fuel degradation and microbiologically influenced corrosion (MIC). The focus of the research presented in this thesis was characterizing the microbial diversity of automotive fuels and automotive fuel environments in the United States via both molecular-based techniques as well as cultivation- based methods in order to gain insight into how this diversity is impacting fuels and fuel system infrastructure. A field survey of fuels including biodiesel, diesel, E10, E85, fuel-grade ethanol and gasoline was conducted; and 454 pyrosequencing of both 16S/18S rRNA genes as well as 16S/18S rRNA (transcribed into cDNA) was applied to identify both total and active microbial communities in these environments. Microbial communities in all fuel types were broadly similar, and prevalent phylotypes included Halomonas spp., Pseudomonas spp., Shewanella spp., Corynebacterium spp. and Acetobacter spp. Pyrosequencing libraries generated from cDNA and DNA indicated that the active and total communities of the sampled environments show significant overlap. The microbial communities of storage tanks containing fuel-grade ethanol and water were also characterized by molecular and cultivation-based techniques. -

Celebrated Turkish-German Actress Meryem Uzerli Speaks to Community Exclusively on Her Launching Pad Muhteşem Yüzyıl and the Journey Beyond
Community Community Noble ‘Labour International Reforms P7School P16 in Qatar: organises a workshop Achievements and ‘Refining of Teaching Next Steps’ discusses Methods’ for its measures taken for faculty members. the welfare of workers. Sunday, April 14, 2019 Sha’baan 9, 1440 AH Doha today: 230 - 330 Hearing Hurrem Celebrated Turkish-German actress Meryem Uzerli speaks to Community exclusively on her launching pad Muhteşem Yüzyıl and the journey beyond. P4-6 COVER STORY QUIZ SHOWBIZ The sinking of Titanic Disney unveils teaser of The Rise of Skywalker. Page 11 Page 15 2 GULF TIMES Sunday, April 14, 2019 COMMUNITY ROUND & ABOUT PRAYER TIME Fajr 3.53am Shorooq (sunrise) 5.14am Zuhr (noon) 11.36am Asr (afternoon) 3.05pm Maghreb (sunset) 5.57pm Isha (night) 7.27pm USEFUL NUMBERS Hellboy Hellboy, caught between the worlds of the supernatural and Emergency 999 DIRECTION:Neil Marshall human, battles an ancient sorceress bent on revenge. Worldwide Emergency Number 112 CAST: David Harbour, Ian McShane, Milla Jovovich Kahramaa – Electricity and Water 991 SYNOPSIS: Based on the graphic novels by Mike Mignola, THEATRES: The Mall, Landmark, Royal Plaza Local Directory 180 International Calls Enquires 150 Hamad International Airport 40106666 Labor Department 44508111, 44406537 Mowasalat Taxi 44588888 Qatar Airways 44496000 Hamad Medical Corporation 44392222, 44393333 Qatar General Electricity and Water Corporation 44845555, 44845464 Primary Health Care Corporation 44593333 44593363 Qatar Assistive Technology Centre 44594050 Qatar News Agency 44450205 44450333 Q-Post – General Postal Corporation 44464444 Humanitarian Services Offi ce (Single window facility for the repatriation of bodies) Ministry of Interior 40253371, 40253372, 40253369 Ministry of Health 40253370, 40253364 Hamad Medical Corporation 40253368, 40253365 Qatar Airways 40253374 Madhura Raja troubles an entire village, the people turn to the only man who DIRECTION: Vysakh can save them: Raja, the fl amboyant don with a heart of gold. -

Science in School
Subscribe free in Europe: FREE www.scienceinschool.org Science in School The European journal for science teachers Winter 2016 | Issue 38 | Issue 2016 Winter Faster,Faster, cheaper,cheaper, CRISPR:CRISPR: thethe newnew genegene technologytechnology revolutionrevolution ISSN: 1818-0353 www.scienceinschool.org ISSN: 1818-0353 www.scienceinschool.org INSPIREINSPIRE EuropeanEuropean CanSatCanSat Published and funded by EIROforum by funded and Published CompetitionCompetition 20162016 TEACHTEACH PracticalPractical pyrotechnicspyrotechnics Image courtesy of Scott Ingram; image source: Flickr Copyright ESA WHAT HAPPENS WHEN CELLS 12 EUROPEAN CANSAT 22 EMBRACE DAMAGE? COMPETITION 2016 Scientists propose a new hypothesis to tackle This June, students from around Europe met in one of the big remaining mysteries in animal Portugal to compete in the European CanSat evolution. competition. One of their teachers tells us more. Image courtesy of Nicola Graf Image courtesy of john_hawn; image source: Flickr of john_hawn; image source: Image courtesy WIND AND RAIN: METEOROLOGY IN THE CLASSROOM 36 Why does it rain? Can we predict it? Give physics students a mass of weather data and Flickr of Thomas Hawk; image source: Image courtesy some information technology, and they can try working this out for themselves. UNDERSTAND INSPIRE 4 News from the EIROs: Proxima b, 22 European CanSat Competition 2016 extremophiles and record-breaking cables 25 Compound Interest: communicating 8 Blended senses: understanding synaesthesia chemistry with engaging graphics 12 -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Revision Guide
Revision Guide Chemistry - Unit 3 Physical and Inorganic Chemistry GCE A Level WJEC These notes have been authored by experienced teachers and are provided as support to students revising for their GCE A level exams. Though the resources are comprehensive, they may not cover every aspect of the specification and do not represent the depth of knowledge required for each unit of work. 1 Content Page Section 2 3.1 – Redox and standard electrode potential 13 3.2 - Redox reactions 20 3.3 - Chemistry of the p-block 30 3.4 - Chemistry of the d-block transition metals 35 3.5 - Chemical kinetics 44 3.6 - Enthalpy changes for solids and solutions 50 3.7 - Entropy and feasibility of reactions 53 3.8 - Equilibrium constants 57 3.9 - Acid-base equilibria 66 Acknowledgements 2 3.1 – Redox and standard electrode potential Redox reactions In AS, we saw that in redox reactions, something is oxidised and something else is reduced (remember OILRIG – this deals with loss and gain of electrons). Another way that we can determine if a redox reaction has happened is by using oxidation states or numbers (see AS revision guide pages 2 and 44). You need to know that: - • oxidation is loss of electrons • reduction is gain of electrons • an oxidising agent is a species that accepts electrons, thereby helping oxidation. It becomes reduced itself in the process. • a reducing agent is a species that donates electrons, thereby helping reduction. It becomes oxidised itself in the process. You also should remember these rules for assigning oxidation numbers in a compound: - 1 All elements have an oxidation number of zero (including diatomic molecules like H2) 2 Hydrogen is 1 unless it’s with a Group 1 metal, then it’s -1 3 Oxygen is -2 (unless it’s a peroxide when it’s -1, or reacted with fluorine, when it’s +2). -

UNESCO Scientific Colloquium on Factors Impacting the Underwater Cultural Heritage (Royal Library of Belgium, Brussels, 13 & 14 December 2011)
UNESCO SCIENTIFIC COLLOQUIUM ON FACTORS IMPACTING UNDERWATER CULTURAL HERITAGE ROYAL LIBRARY OF BELGIUM, BRUSSELS 13 AND 14 DECEMBER 2011 0 1 2 Contents1 1.0 General Context 1.1 The significance of underwater cultural heritage…………………………………………………………5 1.2 The future of underwater archaeology..............................................................................................9 2.0 Commercial exploitation, commercial archaeological interventions and international cooperation 2.1 The extent and the prevention of pillaging on submerged archaeological sites – the French experience.....................................................................................................................................12 2.2 The centenary of the Titanic and the treaty giving legal protection ...............................................17 3.0 Trawling and fishing 3.1 Quantification of trawl damage to pre-modern shipwreck sites: case studies from the Aegean and Black Seas..............................................................................................................................24 4.0 Developing the seabed, resource extraction and renewable energy development at Sea 4.1 The consideration of archaeological sites in oil and gas drilling operations....................................31 4.2 The significance and contribution of marine aggregates.................................................................38 5.0 Environmental impact and climate change 5.1 The appearance of new bacteria (titanic bacterium) and metal corrosion…….................................44 -

Stamp News Canadian
www.canadianstampnews.ca An essential resource for the CANADIAN advanced and beginning collector Like us on Facebook at www.facebook.com/canadianstampnews Follow us on Twitter @trajanpublisher STAMP NEWS Follow us on Instagram @trajan_csn Volume 44 • Number 02 May 14 - 27, 2019 $4.50 ‘Must have’ 1870 Rise of non-traditional themes Small Queen die corresponds with lettermail’s decline By Jesse Robitaille 1851, the Province of Canada issued its This is the first story in a two-part se- first stamp, which was also the world’s proof to highlight ries highlighting the transition away first thematic stamp, this depicting the from traditional philatelic themes and industrious beaver; however, until about towards thematic collecting. the turn of this century, thematic issues Sparks sale Only recently earning the consider- were few and far between. ation of serious philatelists as a legitimate Aside from the first U.S. stamps, By Jesse Robitaille collecting niche, thematic stamps trav- which, unsurprisingly, depict former Described by auctioneers as a elled a hard-fought road before coming presidents Benjamin Franklin and George “must have for any serious to the philatelic forefront with the com- Continued on page 22 Small Queen collector” and a mercialization of the U.S. Postal Service boon to exhibitors of the long- (USPS) about 20 years ago. running series, an 1870 one-cent Of course, Canada is no stranger to Small Queen large die proof is thematic issues. Nearly 170 years ago, in expected to bring $10,000 at auction this May. Traditional themes of U.S. stamps To be offered as Lot 67 of include important historical events Sparks Auctions’ four-session like the American, the 200th “Sale 30,” the black die proof is anniversary of which was marked sunken in and pasted to a card An 1870 one-cent Small Queen large on a 13-cent stamp from the 1977 measuring 51 millimetres by 77 black die proof is expected to bring ‘Bicentennial’ series. -
A Manganate Ester Hydrolysis Mn(IV)
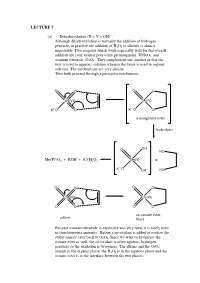
LECTURE 7 (a) Dihydroxylation (X = Y = OH) Although dihydroxylation is formally the addition of hydrogen peroxide, in practice the addition of H2O2 to alkenes is almost impossible. Two reagents which work especially well for this overall addition are cold, neutral potassium permanganate, KMnO4, and osmium tetroxide, OsO4. They complement one another in that the first is used in aqueous solution whereas the latter is used in organic solvents. The mechanisms are very similar. Thus both proceed through a pericyclic mechanism: O O O O Mn(VII) Mn(V) +- K+-O O K O O a manganate ester hydrolysis O OH HO Mn(IV)O2 + KOH + 0.5 H2O2 Mn(V) + +- K O OH HO O O O O Os(VIII) Os(VI) O O O O an osmate ester, yellow black Because osmium tetroxide is expensive and very toxic it is rarely used in stoichiometric amounts. Rather a co-oxidant is added to oxidise the stable osmate ester back to OsO4. Since we want to hydrolyse the osmate ester as well, the co-oxidant is often aqueous hydrogen peroxide so the oxidation is two-phase. The alkene and the OsO4 remain in the organic phase, the H2O2 is in the aqueous phase and the osmate ester is at the interface between the two phases: H OH H OH H OsO4, + aq.H2O2, CH Cl H 2 2 OH H H OH (c) Epoxidation (X = Y = O) The reaction of alkenes with peroxy acids (RCO3H) leads to a cyclic ether, known as an epoxide, as follows: R R O O O H O H O O Although the reaction will work with peroxyacetic acid (R = Me) it works best with peroxy acids bearing electron-withdrawing groups e.g. -

Upcoming Events Monthly Profiles Happenings at IGB Image of the Month IP @ IGB Administrative News
Upcoming Events Image Of The Month IGB Monthly Profiles NEWS IP @ IGB Happenings at IGB Administrative News Volume 7, Number 1 UPCOMING EVENTS FEATURED NEWS IMAGE OF THE MONTH Institute for Universal Biology 2 (IUB) - NASA Astrobiology Institute Seminar Lecture Series The Art of Yellowstone Science: Mammoth Hot Springs as a Window on Evolutionary Processes February 14, 2014, 12:00 p.m. 612 Institute for Genomic Biology Genomics for Judges: Bruce W. Fouke Educating Judges on DNA Director, Roy J. Carver Biotechnology Center Departments of Geology & Microbiology University of Illinois at Urbana-Champaign 3 IGB Seminar (BCXT) Chemical Disequilibrium, Hydrothermal Vents, and the Origin of Metabolism February 18, 2014, 12:00 p.m. 612 Institute for Genomic Biology Laurie M. Barge, PhD Profile: Jet Propulsion Laboratory May Berenbaum California Institute of Technology The Center for Advanced Study 4 Twenty-Third Annual Lecture Me to We: Searching for the Genetic Roots of Sociality February 19, 2014, 7:30 p.m. This month’s image, “Laser Capture Knight Auditorium, Spurlock Museum and Microdissection of Sorghum Roots,” shows root tips of sorghum plants treated Gene E. Robinson with aluminum. Researchers used lasers Director, Institute for Genomic Biology Microbes Dominate Deep to dissect out specific types of cells and University of Illinois at Urbana-Champaign Sandstone Formations tissues in treated plants in order to study the plant’s response to toxicity. IGB Seminar (ReBTE) This image was created using the Veritas 5 laser capture microdissection and laser Engineered Microenvironments for Probing cutting system, and is provided courtesy Cell Fate Decisions of Mayandi Sivaguru of Core Facilities. -

Take a Dive to See the Remains of the Titanic Starting in 2018, an Expedition to the Final Resting Place of the Ill-Fated Luxury Liner Will Cost You $105,129
MNN.com > Tech > Research & Innovations Take a dive to see the remains of the Titanic Starting in 2018, an expedition to the final resting place of the ill-fated luxury liner will cost you $105,129. MICHAEL D'ESTRIES March 22, 2017, 10:44 a.m. 1 In the race to digitally map the wreck of the Titanic in great detail before it disappears, researchers are turning to tourism to help off-set expedition costs. (Photo: OceanGate, Inc. ) More than a century after it slipped under the waves at 2:20 a.m. on April 15, 1912, the RMS Titanic remains a constant object of fascination, intrigue and ever-evolving legend. Unfortunately for those determined to solve the mystery behind her ill-fated maiden voyage, the window of opportunity to study what remains of the ship will soon come to a close. According to a 2016 study, what remains of the Titanic will likely be little more than a rust stain on the ocean floor by 2030. This rapid deterioration is due to the presence of a unique species of bacteria, Halomonas titanicae, that feeds vociferously on the ship's steel. "We tend to have this idea that these wrecks are time capsules frozen in time, when in fact there all kinds of complex ecosystems feeding off them, even at the bottom of that great dark ocean," Dan Conlin, curator of maritime history at the Maritime Museum of the Atlantic in Halifax told Live Science in 2010. With the clock ticking on the Titanic appearance as a ship and not a collapsed mass of rust, researchers are preparing a series of scientific expeditions to the site starting in 2018. -

The Anodic Oxidation of Maleic Acid
Scholars' Mine Masters Theses Student Theses and Dissertations 1966 The anodic oxidation of maleic acid Larry D. Gilmartin Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Chemical Engineering Commons Department: Recommended Citation Gilmartin, Larry D., "The anodic oxidation of maleic acid" (1966). Masters Theses. 5776. https://scholarsmine.mst.edu/masters_theses/5776 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. THE ANODIC OXIDATION OF MALEIC ACID BY LARRY D. GILMARTIN A THESIS submitted to the faculty of THE UNIVERSITY OF MISSOURI AT ROLLA in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE IN CHEMICAL ENGINEERING Rolla, Missouri 1966 Approved by (advisor) THE ANODIC OXIDATION OF MALEIC ACID Larry D. Gilmartin ABSTRACT The purpose of this investigation was to determine the mechanism of the anodic oxidation of maleic acid on platinized-platinum electrodes at 80°C. Current density-potential studies were conducted varying the parameters of maleic acid concentration and pH. The faradaic efficiency of the oxidation of maleic acid to yield co 2 was determined. The effect of temperature on current density was also studied to determine the activation energy for the reaction. The oxidation of maleic acid occurred only in acidic solutions. The faradaic efficiency was found to be ap proximately 97 ± 5 per cent. A linear Tafel region was found which had a slope of 145 - 170 millivolts <~ 2.3RT/aF).