About That Atomic Weight
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -

No. It's Livermorium!
in your element Uuh? No. It’s livermorium! Alpha decay into flerovium? It must be Lv, saysKat Day, as she tells us how little we know about element 116. t the end of last year, the International behaviour in polonium, which we’d expect to Union of Pure and Applied Chemistry have very similar chemistry. The most stable A(IUPAC) announced the verification class of polonium compounds are polonides, of the discoveries of four new chemical for example Na2Po (ref. 8), so in theory elements, 113, 115, 117 and 118, thus Na2Lv and its analogues should be attainable, completing period 7 of the periodic table1. though they are yet to be synthesized. Though now named2 (no doubt after having Experiments carried out in 2011 showed 3 213 212m read the Sceptical Chymist blog post ), that the hydrides BiH3 and PoH2 were 9 we shall wait until the public consultation surprisingly thermally stable . LvH2 would period is over before In Your Element visits be expected to be less stable than the much these ephemeral entities. lighter polonium hydride, but its chemical In the meantime, what do we know of investigation might be possible in the gas their close neighbour, element 116? Well, after phase, if a sufficiently stable isotope can a false start4, the element was first legitimately be found. reported in 2000 by a collaborative team Despite the considerable challenges posed following experiments at the Joint Institute for by the short-lived nature of livermorium, EMMA SOFIA KARLSSON, STOCKHOLM, SWEDEN STOCKHOLM, KARLSSON, EMMA SOFIA Nuclear Research (JINR) in Dubna, Russia. -

The Quest to Explore the Heaviest Elements Raises Questions About How Far Researchers Can Extend Mendeleev’S Creation
ON THE EDGE OF THE PERIODIC TABLE The quest to explore the heaviest elements raises questions about how far researchers can extend Mendeleev’s creation. BY PHILIP BALL 552 | NATURE | VOL 565 | 31 JANUARY 2019 ©2019 Spri nger Nature Li mited. All ri ghts reserved. ©2019 Spri nger Nature Li mited. All ri ghts reserved. FEATURE NEWS f you wanted to create the world’s next undiscovered element, num- Berkeley or at the Joint Institute for Nuclear Research (JINR) in Dubna, ber 119 in the periodic table, here’s a possible recipe. Take a few Russia — the group that Oganessian leads — it took place in an atmos- milligrams of berkelium, a rare radioactive metal that can be made phere of cold-war competition. In the 1980s, Germany joined the race; I only in specialized nuclear reactors. Bombard the sample with a beam an institute in Darmstadt now named the Helmholtz Center for Heavy of titanium ions, accelerated to around one-tenth the speed of light. Ion Research (GSI) made all the elements between 107 and 112. Keep this up for about a year, and be patient. Very patient. For every The competitive edge of earlier years has waned, says Christoph 10 quintillion (1018) titanium ions that slam into the berkelium target Düllmann, who heads the GSI’s superheavy-elements department: — roughly a year’s worth of beam time — the experiment will probably now, researchers frequently talk to each other and carry out some produce only one atom of element 119. experiments collaboratively. The credit for creating later elements On that rare occasion, a titanium and a berkelium nucleus will collide up to 118 has gone variously, and sometimes jointly, to teams from and merge, the speed of their impact overcoming their electrical repul- sion to create something never before seen on Earth, maybe even in the Universe. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Download Article (PDF)
See also www.iupac.org/ See also www.iupac.org/ publications/ci/indexes/stamps.html publications/ci/indexes/stamps.html StampsStampsHed InternationalInternational Oganesson, Where Art Thou? n a press release dated 30 December 2015, the International Union of Pure and Applied Chemistry I(IUPAC) announced that a thorough review by independent experts of the experimental data avail- able for the syntheses of elements 113, 115, 117, and 118 has been concluded, and that the discovery of the four elements completing the 7th row of the periodic table was confirmed. The elemental names and sym- bols proposed shortly thereafter by the corresponding of Armenian descent and the scientifi c leader of the discovery teams met the criteria prescribed by IUPAC Flerov Laboratory of Nuclear Reactions at the Joint In- for naming new elements, and nihonium (Nh), mos- stitute for Nuclear Research in Dubna, Russia, where covium (Mc), tennessine (Ts), and oganesson (Og), dozens of new superheavy isotopes have been made. became permanent within a few months. As such, the Interestingly, oganesson is only the second chemical ending of the name of element 118 and its location in element to be named after a living person since the the periodic table, below radon in group 18, are con- name of element 106 (seaborgium, Sg) was confi rmed sistent with the assumption that oganesson could be by IUPAC in 1997, when the legendary American chem- regarded as a noble gas. ist Glenn Seaborg (1912-1999) was still alive. The stamp also shows the californium-249 and calcium-48 iso- However, the electronic structure of superheavy el- topes involved in the production of oganesson-294, ements, and consequently their physical and chemi- and the radioactive decay chain of Og-294 that leads cal properties, is signifi cantly aff ected by relativistic to the transient generation of livermorium-290, fl erovi- eff ects, even more so than the ones often invoked to um-286, and copernicium-282. -
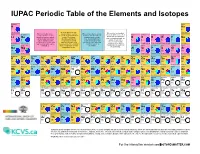
IUPAC Periodic Table of the Elements and Isotopes
IUPAC Periodic Table of the Elements and Isotopes hydrogen helium H He 1 2 1.008 Element has two or more [1.007 84, 1.008 11] Element has no standard 4.002 602(2) Element has two or more isotopes that are used to Element has only one isotope lithium beryllium atomic weight because all of boron carbon nitrogen oxygen fluorine neon isotopes that are used to determine its standard atomic that is used to determine its its isotopes are radioactive determine its atomic weight. weight. The isotopic standard atomic weight. Li Be and, in normal materials, no B C N O F Ne Variations are well known, abundance and atomic Thus, the standard atomic isotope occurs with a 3 4 and the standard atomic weights vary in normal weight is invariant and is 5 6 7 8 9 10 6.94 characteristic isotopic 10.81 12.011 14.007 15.999 weight is given as lower and materials, but upper and given as a single value with [6.938, 6.997] 9.012 1831(5) abundance from which a [10.806, 10.821] [12.0096, 12.0116] [14.006 43, 14.007 28] [15.999 03, 15.999 77] 18.998 403 163(6) 20.1797(6) upper bounds within square lower bounds of the standard an IUPAC evaluated standard atomic weight can sodium magnesium brackets, [ ]. atomic weight have not been uncertainty. aluminium silicon phosphorus sulfur chlorine argon be determined. Na Mg assigned by IUPAC. Al Si P S Cl Ar 11 12 13 14 15 16 17 18 24.305 28.085 32.06 35.45 39.95 22.989 769 28(2) [24.304, 24.307] 26.981 5385(7) [28.084, 28.086] 30.973 761 998(5) [32.059, 32.076] [35.446, 35.457] [39.792, 39.963] potassium calcium scandium titanium -

Beyond Oganesson: Relativistic Effects and the Next Row of the Periodic Table
Joseph C. Wright CHEM 545 Seminar Date: Nov. 20th Beyond Oganesson: Relativistic Effects and the Next Row of the Periodic Table There is no more ubiquitous—or more instantly recognizable—embodiment of modern chemistry than the periodic table of the elements. The number of chemical elements known has expanded enormously from the 11 of antiquity (C, S, Fe, Cu, As, Ag, Sn, Sb, Au, Hg, and Pb) to 118 today, with elements 119 and 120 having been reported but not yet confirmed unequivocally. Since the pioneering work of H. Moseley at the beginning of the 20th century, atomic number Z has been recognized as the table’s fundamental “index” and lies at the heart of the periodic law: when the elements are arranged in order of increasing atomic number, those possessing similar properties appear at regular intervals. But will it always be so? Even as we celebrate the sesqui- centennial of Dmitri Mendeleev’s seminal contributions, theoretical predictions are converging to suggest that the enlarged periodic table of the not-too-distant future will look quite different. Foundational to such predictions is (special) relativity. An electron in the vicinity of the nucleus is accelerated to a speed v ever nearer that of light as Z increases, which results in an ef- fectively increased electron mass calculable in terms of the electron rest mass m0 as m0 m' = . 1 (v/c)2 The consequences of this relativistic mass increase� − are most easily appreciated via consideration of hydrogen-like species. For a one-electron system, the correspondingly modified Bohr radius 2 4πε0ℏ a0' = m'Ze2 implies a relativistically contracted orbital.1 The effect is most pronounced for s orbitals (ℓ = 0) as only they give rise to nonvanishing electron density at the nucleus, at least in Schrödinger’s treatment. -

IUPAC Is Naming the Four New Elements Nihonium, Moscovium, Tennessine, and Oganesson
Advancing Chemistry Worldwide President Vice President Secretary General Prof. Natalia P. Tarasova (Russia) Prof. Qi-Feng Zhou (China) Prof. Richard Hartshorn (New Zealand) Past President Treasurer Executive Director Dr. Mark C. Cesa (USA) Mr. Colin J. Humphris (UK) Dr. Lynn M. Soby (USA) For Release 8 June 2016 IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson Following on the earlier reports that the claims for discovery of these elements have been fulfilled [1, 2], the discoverers have been invited to propose names and the following are now disclosed for public review: • Nihonium and symbol Nh, for the element 113, • Moscovium and symbol Mc, for the element 115, • Tennessine and symbol Ts, for the element 117, and • Oganesson and symbol Og, for the element 118. The IUPAC Inorganic Chemistry Division has reviewed and considered these proposals and recommends these for acceptance. A five-month public review is now set, prior to the formal approval by IUPAC Council. The guidelines for the naming the elements were recently revised [3] and shared with the discoverers to assist in their proposals. Keeping with tradition, newly discovered elements can be named after: (a) a mythological concept or character (including an astronomical object), (b) a mineral or similar substance, (c) a place, or geographical region, (d) a property of the element, or (e) a scientist. The names of all new elements in general would have an ending that reflects and maintains historical and chemical consistency. This would be in general “-ium” for elements belonging to groups 1-16, “-ine” for elements of group 17 and “-on” for elements of group 18. -
Chinese Names of New Elements with Z = 113, 115, 117 &
核データニュース,No.118 (2017) 話題・解説(I) Chinese Names of New Elements with Z = 113, 115, 117 & 118 Shan-Gui Zhou (周善贵/周善貴)1 Institute of Theoretical Physics, Chinese Academy of Sciences, Beijing 100190, China (中国科学院理论物理研究所/中國科學院理論物理研究所) [email protected] ――――――――――――――――――――――――――――――――――――――― 1. Introduction After the 4th Joint IUPAC/IUPAP Working Party confirmed the discovery of the elements with Z = 113, 115, 117 and 118 [1], the naming process of these new elements was officially started. In the end of November 2016, IUPAC announced the new names and symbols: nihonium (Nh) for element 113, moscovium (Mc) for element 115, tennessine (Ts) for element 117 and oganesson (Og) for element 118 [2]. In July 2017, the Council of the IUPAC ratified these names and symbols [3]. Several tens of languages have been used to translate element names [4,5]. In most of these languages, Latin scripts (letters) or native writing systems (syllable characters), e.g., Katakana in Japanese, are used for the element names. However, we use the Chinese characters or scripts (Kanji) in China [6]. In 1932, the Ministry of Education of China announced the Chinese names for 89 elements, covering the elements from hydrogen to uranium with exceptions for astatine, francium and protactinium [7]. Since then, more than ten announcements have been officially made concerning Chinese names of the elements. Although there were some strong objections against creating new characters and there were also some different proposals concerning how to represent an element in Chinese [7], e.g., to simply use Latin names, to use phonetic transcription or to use two or more characters, now it has been well established that one Chinese character should be chosen or, if necessary, created for an element. -
The Periodic Table of Elements
The Periodic Table of Elements 1 2 H 6 Atomic Number = Number of Protons= Number of Electrons He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons+ Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og FRANCIUM RADIUM RUTHERFORDIUM -

On the Discovery of New Elements (IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability.