Oganesson Periodic Table of Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
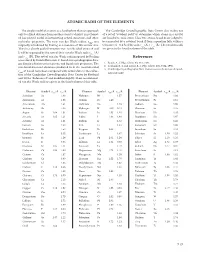
ATOMIC RADII of the ELEMENTS References
ATOMIC RADII OF THE ElEMENTS The simple model of an atom as a hard sphere that can approach The Cambridge Crystallographic Data Center also makes use only to a fixed distance from another atom to which it is not bond- of a set of “covalent radii” to determine which atoms in a crystal ed has proved useful in interpreting crystal structures and other are bonded to each other . Thus two atoms A and B are judged to molecular properties . The term van der Waals radius, rvdw, was be connected by a covalent bond if their separation falls within a originally introduced by Pauling as a measure of this atomic size . tolerance of ±0 .4 Å of the sum rcov (A) + rcov (B) . The covalent radii Thus in a closely packed structure two non-bonded atoms A and are given in the fourth column of the table . B will be separated by the sum of their van der Waals radii rvdw (A) and rvdw (B) . The set of van der Waals radii proposed by Pauling References was refined by Bondi (Reference 1) based on crystallographic data, gas kinetic collision cross sections, and liquid state properties . The 1 . Bondi, A ., J. Phys. Chem. 68, 441, 1964 . non-bonded contact distances predicted from the recommended 2 . Rowland, R . S . and Taylor, R ., J. Phys. Chem. 100, 7384, 1996 . 3 . Cambridge Crystallographic Data Center, www .ccdc .cam .ac .uk/prod- r of Bondi have been compared with actual data in the collec- vdw ucts/csd/radii/ tion of the Cambridge Crystallographic Data Center by Rowland and Taylor (Reference 2) and modified slightly . -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -

Chapter 7 Electron Configuration and the Periodic Table
Chapter 7 Electron Configuration and the Periodic Table Copyright McGraw-Hill 2009 1 7.1 Development of the Periodic Table • 1864 - John Newlands - Law of Octaves- every 8th element had similar properties when arranged by atomic masses (not true past Ca) • 1869 - Dmitri Mendeleev & Lothar Meyer - independently proposed idea of periodicity (recurrence of properties) Copyright McGraw-Hill 2009 2 • Mendeleev – Grouped elements (66) according to properties – Predicted properties for elements not yet discovered – Though a good model, Mendeleev could not explain inconsistencies, for instance, all elements were not in order according to atomic mass Copyright McGraw-Hill 2009 3 • 1913 - Henry Moseley explained the discrepancy – Discovered correlation between number of protons (atomic number) and frequency of X rays generated – Today, elements are arranged in order of increasing atomic number Copyright McGraw-Hill 2009 4 Periodic Table by Dates of Discovery Copyright McGraw-Hill 2009 5 Essential Elements in the Human Body Copyright McGraw-Hill 2009 6 The Modern Periodic Table Copyright McGraw-Hill 2009 7 7.2 The Modern Periodic Table • Classification of Elements – Main group elements - “representative elements” Group 1A- 7A – Noble gases - Group 8A all have ns2np6 configuration(exception-He) – Transition elements - 1B, 3B - 8B “d- block” – Lanthanides/actinides - “f-block” Copyright McGraw-Hill 2009 8 Periodic Table Colored Coded By Main Classifications Copyright McGraw-Hill 2009 9 Copyright McGraw-Hill 2009 10 • Predicting properties – Valence -

Chemistry – Inorganic Chemistry
Answer on Question #53306 – Chemistry – Inorganic Chemistry Question What is oxidation state? How can find out the oxidation state of particular element? Explain its trend in the group and period, give reasons Answer The oxidation state is an indicator of the degree of oxidation (loss of electrons) of an atom in a chemical compound. The oxidation state, which may be positive, negative or equal to zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were completely ionic, with no covalent component. To find out the oxidation state of particular element one should use some simple rules: 1. The oxidation state of an element in a simple substance (for example, He or Cl2, or Fe, or C, or whatever containing one type of atoms) is equal to zero. 2. The sum of the oxidation states of all the atoms or ions in a neutral compound is zero. 3. The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion. 4. The more electronegative element in a substance is given a negative oxidation state. The less electronegative one is given a positive oxidation state. 5. Some elements almost always have the same oxidation states in their compounds: Element Oxidation state Group 1 metals (Li, Na, K, Rb, Cs, Fr) always +1 Group 2 metals (Be, Mg, Ca, Sr, Ba, Ra) always +2 Fluorine (F) always -1 Oxygen (O) usually -2 (except in peroxides (-1) and F2O (+2)) Hydrogen (H) usually +1 (except in metal hydrides (-1)) Having known the oxidation states of these elements in the compound and having known the rule 3, the oxidation state of particular element can be found. -

Electron Configuration Example Script
Electron Configuration Example Script This video demonstrates how to write electron configurations and draw orbital diagrams for main group elements. To write an electron configuration you could memorize the order in which orbitals are filled according to their energy level, but a more convenient method is to use the periodic table. The periodic table is arranged in blocks, each block represents an orbital, and each space in the block counts as one electron. The s block is the first two left-hand columns of the periodic table and includes helium. The p block is the last six columns on the right hand side stating at boron. The d block is the transition metals in the middle of the periodic table, and the f block is the lanthanide and actinide series. To begin start at the top left hand corner of the periodic table and work your way down by reading across a row from left to right and filling in the proper amount of electrons for each orbital until you reach your element. Use the number assigned to each row, 1 thru 7, as the value of the principle quantum number n, when you arrive at the d and f blocks; subtract one from the n value for the d orbitals, and two from the n value for the f orbitals. This periodic table has the electron configuration for each row written along the left hand side using the method just outlined. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. -

About That Atomic Weight
BOOKS & ARTS All about that atomic weight creating and proving the existence of these ele- Shaughnessy was involved in the discovery of ments, the stories in the book almost certainly all the recently discovered elements, and was would have been rejected as implausible fiction also the undisputed champion of the unoffi- were they included in a script for a blockbuster cial LLNL contest to see who could watch Star movie on the Transfermium Wars. Wars: The Force Awakens the most times in Chapman’s book is part science, part his- theatres. These stories will undoubtedly make tory, part multi-subject biography told chrono- Superheavy more accessible to audiences with logically with his travelogue interspersed limited knowledge of nuclear chemistry, such throughout. When he reaches Berkeley, he as high school and college chemistry students. realizes, as I once did, that one should pack a Although Chapman demonstrates a deep sweatshirt or two when visiting the Bay Area knowledge of science fiction, one might expect during the summer. Chapman also makes stops the next meeting of the Avengers to be a little in Sweden, Japan, Italy and several other coun- awkward when Thor cites Chapman’s asser- tries to trace the steps of the element hunters tion that thorium is the only comic book char- who pushed the limits of nuclear chemistry to acter to appear on the periodic table. Captain fill in the periodic table. In particular, Chapman America and Iron Man might disagree with Superheavy: Making and Breaking the spends time at the Joint Institute for Nuclear that claim. Chapman should be prepared as Periodic Table Research in Dubna, Russia, interviewing Yuri Titanium Man, Cobalt Man and all the Metal Edited by Kit Chapman Oganessian, who lends his name to element Men also might pay him a visit in the not too Bloomsbury Sigma, 2019, 304pp, £16.99. -
![Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N](https://docslib.b-cdn.net/cover/8398/atomic-and-ionic-radii-of-elements-1-96-martinrahm-a-roald-hoffmann-a-and-n-668398.webp)
Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N
DOI:10.1002/chem.201602949 Full Paper & Elemental Radii Atomic and Ionic Radii of Elements 1–96 MartinRahm,*[a] Roald Hoffmann,*[a] and N. W. Ashcroft[b] Abstract: Atomic and cationic radii have been calculated for tive measureofthe sizes of non-interacting atoms, common- the first 96 elements, together with selected anionicradii. ly invoked in the rationalization of chemicalbonding, struc- The metric adopted is the average distance from the nucleus ture, and different properties. Remarkably,the atomic radii where the electron density falls to 0.001 electrons per bohr3, as defined in this way correlate well with van der Waals radii following earlier work by Boyd. Our radii are derived using derived from crystal structures. Arationalizationfor trends relativistic all-electron density functional theory calculations, and exceptionsinthose correlations is provided. close to the basis set limit. They offer asystematic quantita- Introduction cule,[2] but we prefer to follow through with aconsistent pic- ture, one of gauging the density in the atomic groundstate. What is the size of an atom or an ion?This question has been The attractivenessofdefining radii from the electron density anatural one to ask over the centurythat we have had good is that a) the electron density is, at least in principle, an experi- experimental metricinformation on atoms in every form of mental observable,and b) it is the electron density at the out- matter,and (more recently) reliable theory for thesesame ermost regionsofasystem that determines Pauli/exchange/ atoms. And the momentone asks this question one knows same-spinrepulsions, or attractive bondinginteractions, with that there is no unique answer.Anatom or ion coursing down achemical surrounding. -

ARC: an Open-Source Library for Calculating Properties of Alkali Rydberg Atoms
ARC: An open-source library for calculating properties of alkali Rydberg atoms N. Šibalic´a,∗, J. D. Pritchardb, C. S. Adamsa, K. J. Weatherilla aJoint Quantum Center (JQC) Durham-Newcastle, Department of Physics, Durham University, South Road, Durham, DH1 3LE, United Kingdom bDepartment of Physics, SUPA, University of Strathclyde, 107 Rottenrow East, Glasgow, G4 0NG, United Kingdom Abstract We present an object-oriented Python library for computation of properties of highly-excited Rydberg states of alkali atoms. These include single-body effects such as dipole matrix elements, excited-state lifetimes (radiative and black-body limited) and Stark maps of atoms in external electric fields, as well as two-atom interaction potentials accounting for dipole and quadrupole coupling effects valid at both long and short range for arbitrary placement of the atomic dipoles. The package is cross-referenced to precise measurements of atomic energy levels and features extensive documentation to facilitate rapid upgrade or expansion by users. This library has direct application in the field of quantum information and quantum optics which exploit the strong Rydberg dipolar interactions for two-qubit gates, robust atom-light interfaces and simulating quantum many-body physics, as well as the field of metrology using Rydberg atoms as precise microwave electrometers. Keywords: Alkali atom, Matrix elements, Dipole-dipole interactions, Stark shift, Förster resonances PROGRAM SUMMARY They are a flourishing field for quantum information process- Program Title: ARC: Alkali Rydberg Calculator ing [1, 2] and quantum optics [3, 4, 5] in the few to single exci- Licensing provisions: BSD-3-Clause tation regime, as well as many-body physics [6, 7, 8, 9, 10, 11], Programming language: Python 2.7 or 3.5, with C extension in the many-excitations limit. -

Electron Configurations, Orbital Notation and Quantum Numbers
5 Electron Configurations, Orbital Notation and Quantum Numbers Electron Configurations, Orbital Notation and Quantum Numbers Understanding Electron Arrangement and Oxidation States Chemical properties depend on the number and arrangement of electrons in an atom. Usually, only the valence or outermost electrons are involved in chemical reactions. The electron cloud is compartmentalized. We model this compartmentalization through the use of electron configurations and orbital notations. The compartmentalization is as follows, energy levels have sublevels which have orbitals within them. We can use an apartment building as an analogy. The atom is the building, the floors of the apartment building are the energy levels, the apartments on a given floor are the orbitals and electrons reside inside the orbitals. There are two governing rules to consider when assigning electron configurations and orbital notations. Along with these rules, you must remember electrons are lazy and they hate each other, they will fill the lowest energy states first AND electrons repel each other since like charges repel. Rule 1: The Pauli Exclusion Principle In 1925, Wolfgang Pauli stated: No two electrons in an atom can have the same set of four quantum numbers. This means no atomic orbital can contain more than TWO electrons and the electrons must be of opposite spin if they are to form a pair within an orbital. Rule 2: Hunds Rule The most stable arrangement of electrons is one with the maximum number of unpaired electrons. It minimizes electron-electron repulsions and stabilizes the atom. Here is an analogy. In large families with several children, it is a luxury for each child to have their own room. -

Chalcogen-Nitrogen Bond: Insights Into a Key Chemical Motif
Proceedings Chalcogen-nitrogen Bond: Insights into A Key Chemical Motif Marco Bortoli,1 Andrea Madabeni,1 Pablo Andrei Nogara,2 Folorunsho B. Omage,2 Giovanni Ribaudo,3 Davide Zeppilli,1 Joao Batista Teixeira Rocha,2* Laura Orian1* 1 Dipartimento di Scienze Chimiche Università degli Studi di Padova Via Marzolo 1 35131 Padova, Italy; [email protected] (M.B.); [email protected] (A.M..); [email protected] (D.Z.) 2 Departamento de Bioquímica e Biologia Molecular, Universidade Federal de Santa Maria, Santa Maria, 97105-900, RS Brazil; [email protected] (P.A.N.); [email protected] (F.B.O.) 3 Dipartimento di Medicina Molecolare e Traslazionale, Università degli Studi di Brescia, Viale Europa 11, 25123 Brescia, Italy; [email protected] (G.R.) * Correspondence: : [email protected] (J.B.T.R), [email protected] (L.O.); † Presented at the 1st International Electronic Conference on Catalysis Sciences, 10–30 November 2020; Available online: https://eccs2020.sciforum.net/ Published: 10 November 2020 Abstract: Chalcogen-nitrogen chemistry deals with systems in which sulfur, selenium or tellurium is linked to a nitrogen nucleus. This chemical motif is a key component of different functional structures, ranging from inorganic materials and polymers to rationally designed catalysts, to bioinspired molecules and enzymes. The formation of a selenium-nitrogen bond, and its disruption, are rather common events in organic Se-catalyzed processes. In nature, along the mechanistic path of glutathione peroxidase, evidence of the formation of a Se-N bond in highly oxidizing conditions has been reported and interpreted as a strategy to protect the selenoenzyme from overoxidation. -

No. It's Livermorium!
in your element Uuh? No. It’s livermorium! Alpha decay into flerovium? It must be Lv, saysKat Day, as she tells us how little we know about element 116. t the end of last year, the International behaviour in polonium, which we’d expect to Union of Pure and Applied Chemistry have very similar chemistry. The most stable A(IUPAC) announced the verification class of polonium compounds are polonides, of the discoveries of four new chemical for example Na2Po (ref. 8), so in theory elements, 113, 115, 117 and 118, thus Na2Lv and its analogues should be attainable, completing period 7 of the periodic table1. though they are yet to be synthesized. Though now named2 (no doubt after having Experiments carried out in 2011 showed 3 213 212m read the Sceptical Chymist blog post ), that the hydrides BiH3 and PoH2 were 9 we shall wait until the public consultation surprisingly thermally stable . LvH2 would period is over before In Your Element visits be expected to be less stable than the much these ephemeral entities. lighter polonium hydride, but its chemical In the meantime, what do we know of investigation might be possible in the gas their close neighbour, element 116? Well, after phase, if a sufficiently stable isotope can a false start4, the element was first legitimately be found. reported in 2000 by a collaborative team Despite the considerable challenges posed following experiments at the Joint Institute for by the short-lived nature of livermorium, EMMA SOFIA KARLSSON, STOCKHOLM, SWEDEN STOCKHOLM, KARLSSON, EMMA SOFIA Nuclear Research (JINR) in Dubna, Russia.