IUPAC Is Naming the Four New Elements Nihonium, Moscovium, Tennessine, and Oganesson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

Classification of Elements and Periodicity in Properties
74 CHEMISTRY UNIT 3 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES The Periodic Table is arguably the most important concept in chemistry, both in principle and in practice. It is the everyday support for students, it suggests new avenues of research to After studying this Unit, you will be professionals, and it provides a succinct organization of the able to whole of chemistry. It is a remarkable demonstration of the fact that the chemical elements are not a random cluster of • appreciate how the concept of entities but instead display trends and lie together in families. grouping elements in accordance to An awareness of the Periodic Table is essential to anyone who their properties led to the wishes to disentangle the world and see how it is built up development of Periodic Table. from the fundamental building blocks of the chemistry, the understand the Periodic Law; • chemical elements. • understand the significance of atomic number and electronic Glenn T. Seaborg configuration as the basis for periodic classification; • name the elements with In this Unit, we will study the historical development of the Z >100 according to IUPAC Periodic Table as it stands today and the Modern Periodic nomenclature; Law. We will also learn how the periodic classification • classify elements into s, p, d, f follows as a logical consequence of the electronic blocks and learn their main configuration of atoms. Finally, we shall examine some of characteristics; the periodic trends in the physical and chemical properties • recognise the periodic trends in of the elements. physical and chemical properties of elements; 3.1 WHY DO WE NEED TO CLASSIFY ELEMENTS ? compare the reactivity of elements • We know by now that the elements are the basic units of all and correlate it with their occurrence in nature; types of matter. -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -

About That Atomic Weight
BOOKS & ARTS All about that atomic weight creating and proving the existence of these ele- Shaughnessy was involved in the discovery of ments, the stories in the book almost certainly all the recently discovered elements, and was would have been rejected as implausible fiction also the undisputed champion of the unoffi- were they included in a script for a blockbuster cial LLNL contest to see who could watch Star movie on the Transfermium Wars. Wars: The Force Awakens the most times in Chapman’s book is part science, part his- theatres. These stories will undoubtedly make tory, part multi-subject biography told chrono- Superheavy more accessible to audiences with logically with his travelogue interspersed limited knowledge of nuclear chemistry, such throughout. When he reaches Berkeley, he as high school and college chemistry students. realizes, as I once did, that one should pack a Although Chapman demonstrates a deep sweatshirt or two when visiting the Bay Area knowledge of science fiction, one might expect during the summer. Chapman also makes stops the next meeting of the Avengers to be a little in Sweden, Japan, Italy and several other coun- awkward when Thor cites Chapman’s asser- tries to trace the steps of the element hunters tion that thorium is the only comic book char- who pushed the limits of nuclear chemistry to acter to appear on the periodic table. Captain fill in the periodic table. In particular, Chapman America and Iron Man might disagree with Superheavy: Making and Breaking the spends time at the Joint Institute for Nuclear that claim. Chapman should be prepared as Periodic Table Research in Dubna, Russia, interviewing Yuri Titanium Man, Cobalt Man and all the Metal Edited by Kit Chapman Oganessian, who lends his name to element Men also might pay him a visit in the not too Bloomsbury Sigma, 2019, 304pp, £16.99. -

No. It's Livermorium!
in your element Uuh? No. It’s livermorium! Alpha decay into flerovium? It must be Lv, saysKat Day, as she tells us how little we know about element 116. t the end of last year, the International behaviour in polonium, which we’d expect to Union of Pure and Applied Chemistry have very similar chemistry. The most stable A(IUPAC) announced the verification class of polonium compounds are polonides, of the discoveries of four new chemical for example Na2Po (ref. 8), so in theory elements, 113, 115, 117 and 118, thus Na2Lv and its analogues should be attainable, completing period 7 of the periodic table1. though they are yet to be synthesized. Though now named2 (no doubt after having Experiments carried out in 2011 showed 3 213 212m read the Sceptical Chymist blog post ), that the hydrides BiH3 and PoH2 were 9 we shall wait until the public consultation surprisingly thermally stable . LvH2 would period is over before In Your Element visits be expected to be less stable than the much these ephemeral entities. lighter polonium hydride, but its chemical In the meantime, what do we know of investigation might be possible in the gas their close neighbour, element 116? Well, after phase, if a sufficiently stable isotope can a false start4, the element was first legitimately be found. reported in 2000 by a collaborative team Despite the considerable challenges posed following experiments at the Joint Institute for by the short-lived nature of livermorium, EMMA SOFIA KARLSSON, STOCKHOLM, SWEDEN STOCKHOLM, KARLSSON, EMMA SOFIA Nuclear Research (JINR) in Dubna, Russia. -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

Walter C. Ermler
Curriculum Vitae Dr. Walter C. Ermler Professor of Chemistry Department of Chemistry, BSE-1.104E University of Texas at San Antonio One UTSA Circle San Antonio, TX 78249-0698 Phone: 210-458-7005 Fax: 210-458-7428 Email: [email protected] Homepage: https://chemistry.utsa.edu/archives/831 Education Doctor of Philosophy (Physical Chemistry), Ohio State University, 1972 Master of Science (Physical Chemistry), Ohio State University, 1970 Bachelor of Science (Chemistry), Northern Illinois University, 1969 Professional Employment History Academic: 1978-present Professor of Chemistry, University of Texas at San Antonio, 2005-present Chair, Department of Chemistry, University of Texas at San Antonio, 2005-2006 Professor of Physics, Stevens Institute of Technology, 1990-2001 Chair, Dept. Chemistry and Chemical Biology, Stevens Institute of Technology, 1998-1999 Professor of Chemistry, Stevens Institute of Technology, 1984-2001 Associate Professor of Chemistry, Stevens Institute of Technology, 1978-83 Federal: 1993-2019 Program Director, National Science Foundation, MPS-CHE-CTMC/REU, 2018-2020 Program Director, National Science Foundation, MPS-CHE, EHR-REC, 2001-2005 Senior Program Manager, U. S. Department of Energy, ASCR, 1993-1996 Postdoctoral Research Associate: 1973-1978 University of California, Berkeley, Department of Chemistry, 1976-1978 (Kenneth S. Pitzer, Priestly Medalist) University of Chicago Departments of Physics and Chemistry, 1973-1976 (Robert S. Mulliken, Nobel Laureate) Awards and Honors Wiley Visiting Scientist Fellow, EMSL, -

The Quest to Explore the Heaviest Elements Raises Questions About How Far Researchers Can Extend Mendeleev’S Creation
ON THE EDGE OF THE PERIODIC TABLE The quest to explore the heaviest elements raises questions about how far researchers can extend Mendeleev’s creation. BY PHILIP BALL 552 | NATURE | VOL 565 | 31 JANUARY 2019 ©2019 Spri nger Nature Li mited. All ri ghts reserved. ©2019 Spri nger Nature Li mited. All ri ghts reserved. FEATURE NEWS f you wanted to create the world’s next undiscovered element, num- Berkeley or at the Joint Institute for Nuclear Research (JINR) in Dubna, ber 119 in the periodic table, here’s a possible recipe. Take a few Russia — the group that Oganessian leads — it took place in an atmos- milligrams of berkelium, a rare radioactive metal that can be made phere of cold-war competition. In the 1980s, Germany joined the race; I only in specialized nuclear reactors. Bombard the sample with a beam an institute in Darmstadt now named the Helmholtz Center for Heavy of titanium ions, accelerated to around one-tenth the speed of light. Ion Research (GSI) made all the elements between 107 and 112. Keep this up for about a year, and be patient. Very patient. For every The competitive edge of earlier years has waned, says Christoph 10 quintillion (1018) titanium ions that slam into the berkelium target Düllmann, who heads the GSI’s superheavy-elements department: — roughly a year’s worth of beam time — the experiment will probably now, researchers frequently talk to each other and carry out some produce only one atom of element 119. experiments collaboratively. The credit for creating later elements On that rare occasion, a titanium and a berkelium nucleus will collide up to 118 has gone variously, and sometimes jointly, to teams from and merge, the speed of their impact overcoming their electrical repul- sion to create something never before seen on Earth, maybe even in the Universe. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Python Module Index 79
mendeleev Documentation Release 0.9.0 Lukasz Mentel Sep 04, 2021 CONTENTS 1 Getting started 3 1.1 Overview.................................................3 1.2 Contributing...............................................3 1.3 Citing...................................................3 1.4 Related projects.............................................4 1.5 Funding..................................................4 2 Installation 5 3 Tutorials 7 3.1 Quick start................................................7 3.2 Bulk data access............................................. 14 3.3 Electronic configuration......................................... 21 3.4 Ions.................................................... 23 3.5 Visualizing custom periodic tables.................................... 25 3.6 Advanced visulization tutorial...................................... 27 3.7 Jupyter notebooks............................................ 30 4 Data 31 4.1 Elements................................................. 31 4.2 Isotopes.................................................. 35 5 Electronegativities 37 5.1 Allen................................................... 37 5.2 Allred and Rochow............................................ 38 5.3 Cottrell and Sutton............................................ 38 5.4 Ghosh................................................... 38 5.5 Gordy................................................... 39 5.6 Li and Xue................................................ 39 5.7 Martynov and Batsanov........................................ -

Download Article (PDF)
See also www.iupac.org/ See also www.iupac.org/ publications/ci/indexes/stamps.html publications/ci/indexes/stamps.html StampsStampsHed InternationalInternational Oganesson, Where Art Thou? n a press release dated 30 December 2015, the International Union of Pure and Applied Chemistry I(IUPAC) announced that a thorough review by independent experts of the experimental data avail- able for the syntheses of elements 113, 115, 117, and 118 has been concluded, and that the discovery of the four elements completing the 7th row of the periodic table was confirmed. The elemental names and sym- bols proposed shortly thereafter by the corresponding of Armenian descent and the scientifi c leader of the discovery teams met the criteria prescribed by IUPAC Flerov Laboratory of Nuclear Reactions at the Joint In- for naming new elements, and nihonium (Nh), mos- stitute for Nuclear Research in Dubna, Russia, where covium (Mc), tennessine (Ts), and oganesson (Og), dozens of new superheavy isotopes have been made. became permanent within a few months. As such, the Interestingly, oganesson is only the second chemical ending of the name of element 118 and its location in element to be named after a living person since the the periodic table, below radon in group 18, are con- name of element 106 (seaborgium, Sg) was confi rmed sistent with the assumption that oganesson could be by IUPAC in 1997, when the legendary American chem- regarded as a noble gas. ist Glenn Seaborg (1912-1999) was still alive. The stamp also shows the californium-249 and calcium-48 iso- However, the electronic structure of superheavy el- topes involved in the production of oganesson-294, ements, and consequently their physical and chemi- and the radioactive decay chain of Og-294 that leads cal properties, is signifi cantly aff ected by relativistic to the transient generation of livermorium-290, fl erovi- eff ects, even more so than the ones often invoked to um-286, and copernicium-282. -
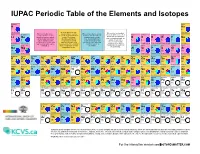
IUPAC Periodic Table of the Elements and Isotopes
IUPAC Periodic Table of the Elements and Isotopes hydrogen helium H He 1 2 1.008 Element has two or more [1.007 84, 1.008 11] Element has no standard 4.002 602(2) Element has two or more isotopes that are used to Element has only one isotope lithium beryllium atomic weight because all of boron carbon nitrogen oxygen fluorine neon isotopes that are used to determine its standard atomic that is used to determine its its isotopes are radioactive determine its atomic weight. weight. The isotopic standard atomic weight. Li Be and, in normal materials, no B C N O F Ne Variations are well known, abundance and atomic Thus, the standard atomic isotope occurs with a 3 4 and the standard atomic weights vary in normal weight is invariant and is 5 6 7 8 9 10 6.94 characteristic isotopic 10.81 12.011 14.007 15.999 weight is given as lower and materials, but upper and given as a single value with [6.938, 6.997] 9.012 1831(5) abundance from which a [10.806, 10.821] [12.0096, 12.0116] [14.006 43, 14.007 28] [15.999 03, 15.999 77] 18.998 403 163(6) 20.1797(6) upper bounds within square lower bounds of the standard an IUPAC evaluated standard atomic weight can sodium magnesium brackets, [ ]. atomic weight have not been uncertainty. aluminium silicon phosphorus sulfur chlorine argon be determined. Na Mg assigned by IUPAC. Al Si P S Cl Ar 11 12 13 14 15 16 17 18 24.305 28.085 32.06 35.45 39.95 22.989 769 28(2) [24.304, 24.307] 26.981 5385(7) [28.084, 28.086] 30.973 761 998(5) [32.059, 32.076] [35.446, 35.457] [39.792, 39.963] potassium calcium scandium titanium