Ununpentium Uup That If Stable Isotopes of Element 115 Can Be Produced
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No. It's Livermorium!
in your element Uuh? No. It’s livermorium! Alpha decay into flerovium? It must be Lv, saysKat Day, as she tells us how little we know about element 116. t the end of last year, the International behaviour in polonium, which we’d expect to Union of Pure and Applied Chemistry have very similar chemistry. The most stable A(IUPAC) announced the verification class of polonium compounds are polonides, of the discoveries of four new chemical for example Na2Po (ref. 8), so in theory elements, 113, 115, 117 and 118, thus Na2Lv and its analogues should be attainable, completing period 7 of the periodic table1. though they are yet to be synthesized. Though now named2 (no doubt after having Experiments carried out in 2011 showed 3 213 212m read the Sceptical Chymist blog post ), that the hydrides BiH3 and PoH2 were 9 we shall wait until the public consultation surprisingly thermally stable . LvH2 would period is over before In Your Element visits be expected to be less stable than the much these ephemeral entities. lighter polonium hydride, but its chemical In the meantime, what do we know of investigation might be possible in the gas their close neighbour, element 116? Well, after phase, if a sufficiently stable isotope can a false start4, the element was first legitimately be found. reported in 2000 by a collaborative team Despite the considerable challenges posed following experiments at the Joint Institute for by the short-lived nature of livermorium, EMMA SOFIA KARLSSON, STOCKHOLM, SWEDEN STOCKHOLM, KARLSSON, EMMA SOFIA Nuclear Research (JINR) in Dubna, Russia. -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Python Module Index 79
mendeleev Documentation Release 0.9.0 Lukasz Mentel Sep 04, 2021 CONTENTS 1 Getting started 3 1.1 Overview.................................................3 1.2 Contributing...............................................3 1.3 Citing...................................................3 1.4 Related projects.............................................4 1.5 Funding..................................................4 2 Installation 5 3 Tutorials 7 3.1 Quick start................................................7 3.2 Bulk data access............................................. 14 3.3 Electronic configuration......................................... 21 3.4 Ions.................................................... 23 3.5 Visualizing custom periodic tables.................................... 25 3.6 Advanced visulization tutorial...................................... 27 3.7 Jupyter notebooks............................................ 30 4 Data 31 4.1 Elements................................................. 31 4.2 Isotopes.................................................. 35 5 Electronegativities 37 5.1 Allen................................................... 37 5.2 Allred and Rochow............................................ 38 5.3 Cottrell and Sutton............................................ 38 5.4 Ghosh................................................... 38 5.5 Gordy................................................... 39 5.6 Li and Xue................................................ 39 5.7 Martynov and Batsanov........................................ -
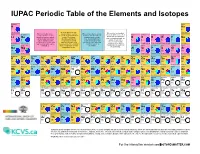
IUPAC Periodic Table of the Elements and Isotopes
IUPAC Periodic Table of the Elements and Isotopes hydrogen helium H He 1 2 1.008 Element has two or more [1.007 84, 1.008 11] Element has no standard 4.002 602(2) Element has two or more isotopes that are used to Element has only one isotope lithium beryllium atomic weight because all of boron carbon nitrogen oxygen fluorine neon isotopes that are used to determine its standard atomic that is used to determine its its isotopes are radioactive determine its atomic weight. weight. The isotopic standard atomic weight. Li Be and, in normal materials, no B C N O F Ne Variations are well known, abundance and atomic Thus, the standard atomic isotope occurs with a 3 4 and the standard atomic weights vary in normal weight is invariant and is 5 6 7 8 9 10 6.94 characteristic isotopic 10.81 12.011 14.007 15.999 weight is given as lower and materials, but upper and given as a single value with [6.938, 6.997] 9.012 1831(5) abundance from which a [10.806, 10.821] [12.0096, 12.0116] [14.006 43, 14.007 28] [15.999 03, 15.999 77] 18.998 403 163(6) 20.1797(6) upper bounds within square lower bounds of the standard an IUPAC evaluated standard atomic weight can sodium magnesium brackets, [ ]. atomic weight have not been uncertainty. aluminium silicon phosphorus sulfur chlorine argon be determined. Na Mg assigned by IUPAC. Al Si P S Cl Ar 11 12 13 14 15 16 17 18 24.305 28.085 32.06 35.45 39.95 22.989 769 28(2) [24.304, 24.307] 26.981 5385(7) [28.084, 28.086] 30.973 761 998(5) [32.059, 32.076] [35.446, 35.457] [39.792, 39.963] potassium calcium scandium titanium -

The Making of Moscovium Yuri Oganessian Relates the Story of the Formation and Decay of a Doubly Odd Moscovium Nucleus
in your element The making of moscovium Yuri Oganessian relates the story of the formation and decay of a doubly odd moscovium nucleus. lement 115 was the first superheavy of the nucleus by two, and we move 288 element with an odd atomic away by two neutrons from the magic 115Mc Enumber (Z) that we synthesized number N = 184. As a result, the nucleus in nuclear reactions using a beam of becomes more stable to alpha decay, 284Nh accelerated 48Ca ions. These experiments 113 but more prone to spontaneous fission were carried out in 2003, on the heels and eventually the chain will be of the first results obtained for even 280 terminated by spontaneous fission. 111Rg elements 114 and 116. We had no At what point will this happen? Only doubts that their odd neighbour 276 experiments will tell. could also be produced in a similar 109Mt In our 2003 experiments, detectors manner; its decay properties however allowed us to document the entire 272 would be very different. 107Bh radioactive family of the nuclei formed, The target isotope 243Am (Z = 95) was and to determine the energy and time of 48 bombarded with ions of Ca (Z = 20) in 268 emission of each alpha particle emitted the hope that a rare process — the fusion 105Dbb as well as the energy of the fragments of 268Rf of these nuclei — would occur. A 291115 104 spontaneous fission. The decay chain of the nucleus did indeed form. During 288115 isotope obtained is pictured. After five the process this nucleus was heated consecutive alpha transitions within the first Credit: Loop Images Ltd / Alamy Stock Photo (to around 4×1011 K), and cooled down 20 seconds, there was a long pause, which through the very fast emission of three brought us a lot of trouble. -

IUPAC Is Naming the Four New Elements Nihonium, Moscovium, Tennessine, and Oganesson
Advancing Chemistry Worldwide President Vice President Secretary General Prof. Natalia P. Tarasova (Russia) Prof. Qi-Feng Zhou (China) Prof. Richard Hartshorn (New Zealand) Past President Treasurer Executive Director Dr. Mark C. Cesa (USA) Mr. Colin J. Humphris (UK) Dr. Lynn M. Soby (USA) For Release 8 June 2016 IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson Following on the earlier reports that the claims for discovery of these elements have been fulfilled [1, 2], the discoverers have been invited to propose names and the following are now disclosed for public review: • Nihonium and symbol Nh, for the element 113, • Moscovium and symbol Mc, for the element 115, • Tennessine and symbol Ts, for the element 117, and • Oganesson and symbol Og, for the element 118. The IUPAC Inorganic Chemistry Division has reviewed and considered these proposals and recommends these for acceptance. A five-month public review is now set, prior to the formal approval by IUPAC Council. The guidelines for the naming the elements were recently revised [3] and shared with the discoverers to assist in their proposals. Keeping with tradition, newly discovered elements can be named after: (a) a mythological concept or character (including an astronomical object), (b) a mineral or similar substance, (c) a place, or geographical region, (d) a property of the element, or (e) a scientist. The names of all new elements in general would have an ending that reflects and maintains historical and chemical consistency. This would be in general “-ium” for elements belonging to groups 1-16, “-ine” for elements of group 17 and “-on” for elements of group 18. -
Chinese Names of New Elements with Z = 113, 115, 117 &
核データニュース,No.118 (2017) 話題・解説(I) Chinese Names of New Elements with Z = 113, 115, 117 & 118 Shan-Gui Zhou (周善贵/周善貴)1 Institute of Theoretical Physics, Chinese Academy of Sciences, Beijing 100190, China (中国科学院理论物理研究所/中國科學院理論物理研究所) [email protected] ――――――――――――――――――――――――――――――――――――――― 1. Introduction After the 4th Joint IUPAC/IUPAP Working Party confirmed the discovery of the elements with Z = 113, 115, 117 and 118 [1], the naming process of these new elements was officially started. In the end of November 2016, IUPAC announced the new names and symbols: nihonium (Nh) for element 113, moscovium (Mc) for element 115, tennessine (Ts) for element 117 and oganesson (Og) for element 118 [2]. In July 2017, the Council of the IUPAC ratified these names and symbols [3]. Several tens of languages have been used to translate element names [4,5]. In most of these languages, Latin scripts (letters) or native writing systems (syllable characters), e.g., Katakana in Japanese, are used for the element names. However, we use the Chinese characters or scripts (Kanji) in China [6]. In 1932, the Ministry of Education of China announced the Chinese names for 89 elements, covering the elements from hydrogen to uranium with exceptions for astatine, francium and protactinium [7]. Since then, more than ten announcements have been officially made concerning Chinese names of the elements. Although there were some strong objections against creating new characters and there were also some different proposals concerning how to represent an element in Chinese [7], e.g., to simply use Latin names, to use phonetic transcription or to use two or more characters, now it has been well established that one Chinese character should be chosen or, if necessary, created for an element. -

International the News Magazine of IUPAC
CHEMISTRY International The News Magazine of IUPAC January-March 2017 Volume 39 No. 1 Chemistry Organizations in a Changing World Woodward’s Words The Periodic Table (Continued?) INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Chemistry International CHEMISTRY International Subscriptions Six issues of Chemistry International (ISSN 0193-6484) will be published quarterly in 2017 (one volume The News Magazine of the International Union of Pure and per annum) in January, Apri, July, and October. The 2017 subscription rate is USD 110.00 for organizations Applied Chemistry (IUPAC) and USD 65.00 for individuals. IUPAC Affiliate Members receive CI as part of their Membership subscrip- tion, and Members of IUPAC bodies receive CI free of charge. All information regarding notes for contributors, subscriptions, Open Access, back volumes and ISSN 0193-6484, eISSN 1365-2192 orders is available online at www.degruyter.com/ci Periodicals postage paid at Durham, NC 27709-9990 and additional mailing offices. POSTMASTER: Send address changes to Chemistry International, IUPAC Secretariat, PO Box 13757, Research Triangle Park, Managing Editor: NC 27709-3757, USA. Fabienne Meyers IUPAC, c/o Department of Chemistry © 2017 International Union of Pure and Applied Chemistry. This work is licensed Boston University under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 Metcalf Center for Science and Engineering International License. 590 Commonwealth Ave. Boston, MA 02215, USA E-mail: [email protected] Front Cover: In a rapidly changing world, what is the fundamental purpose of chemistry organizations, Phone: +1 617 358 0410 what should be their roles and how do they need to refresh themselves to best serve the field and also society at large? Stephen A. -

Status of the Heaviest Elements As of June 2017
Status of the heaviest elements as of June 2017 Don Groom, Particle Data Group Superheavy elements (SHE) are normally synthesized by exposing a high-Z target to a 2.5{7.5 MeV/u ion beam such as 48Ca or 70Zn. The target can be a stable isotope such as 208Pb or 209Bi; in this case the process is called cold fusion. If the target is radioactive (an actinide), it is a \hot fusion" reaction. Targets as heavy as 249Bk and 249Cf have been used. Since fusion is followed by neutron loss (e.g. 248Cf(48Ca,4n)292Lv), the atomic numbers of the beam and target nuclei determine whether the fusion products have even or odd Z. The main laboratories involved are at Dubna (JINR), Darmstadt (GSI), Berkeley (LBNL), and Saitama, Japan (RIKEN) [1]. Fusion cross sections steadily decrease with increasing Z, and are in the picobarn range for the heaviest elements. This means that 1{10 SHE/week can be produced for O(1018) heavy ions on target [1]. In the best of worlds, a superheavy isotope decays via a long chain of α emissions, the last few between well-studied isotopes of known elements. But often the evidence from a given event is unconvincing: Spon- taneous fission may truncate the chain too early, an α might escape the detector before depositing all of its energy, or the decay chain might proceed via previously unknown daughter isotopes. Gold-plated examples are rare, one of these being the observation of 278113 ! 274Rg ! 270Mt ! 266Bh ! 262Db ! 258Lw at RIKEN [2]. The International Union of Pure and Applied Chemistry (IUPAC) gives its blessing to a new element only after its experimental demonstration \beyond reasonable doubt." This usually means convincing re- production of the result at a second laboratory, preferably by a different technique. -

The Search for Superheavy Elements: Historical and Philosophical Perspectives 1. Introduction
1 The Search for Superheavy Elements: Historical and Philosophical Perspectives Helge Kragh* Abstract: The heaviest of the transuranic elements known as superheavy elements (SHE) are produced in nuclear reactions in a few specialized laboratories located in Germany, the U.S., Russia, and Japan. The history of this branch of physical science provides several case studies of interest to the philosophy and sociology of modern science. The story of SHE illuminates the crucial notion of what constitutes a chemical element, what the criteria are for discovering a new element, and how an element is assigned a name. The story also cast light on the sometimes uneasy relationship between physics and chemistry. It is far from obvious that elements with Z > 110 exist in the same sense as oxygen or sodium exists. The answers are not given by nature but by international commissions responsible for the criteria and evaluation of discovery claims. The works of these commissions and of SHE research in general have often been controversial. 1. Introduction Beyond uranium the periodic table includes no less than 26 elements which have all been manufactured in the laboratory and the best known of which is plutonium of atomic number 94. The heaviest of the transuranic elements are often called “superheavy elements” (SHE), a term with no precise meaning but which often refers to the transactinide elements with Z ranging from 103 to 120. In some cases the term is used only for Z > 110. So far the last of the superheavy elements is Z = 118, a substance which received official recognition as an element in 2016 and is named oganesson, chemical symbol Og. -

'Nihonium', 'Moscovium', Among New Periodic Element Names 9 June 2016
'Nihonium', 'moscovium', among new periodic element names 9 June 2016 IUPAC and the International Union of Pure and Applied Physics (IUPAP) bestowed the rights late last year. Synthetic elements are produced through laboratory experiments rather than those found in nature such as hydrogen, carbon or magnesium. IUPAC said in the release Wednesday that newly discovered elements can be named after mythological concepts or characters, minerals or similar substance, places, or geographical regions, a property of the element, or a scientist. Besides being the first element on the periodic Japanese scientist Kosuke Morita (left) and Science table to be discovered and named by Japanese Minister Hiroshi Hase point to the periodic table scientists, element 113 is also Asia's first, displaying the new element 113, during at a press according to Riken, the Japanese state-backed conference in Wako, Saitama prefecture, on June 9, research institute that discovered it, and IUPAC. 2016 While generally welcomed, some thought the Japanese name might be lost on foreigners. Four new elements have been added to the "If you say 'NIHONIUM,' 'Nippon (Nihon)' is periodic table after discoveries by Japanese, something which only the Japanese people use Russian and US scientists, an international and won't be understood by foreigners….", read a authority said, with the new substances including Japanese-language tweet. Asia's first entry on the chart. "Nippon" is a slightly more formal variant of the Scientists in Japan who discovered element 113 country's name in Japanese. have chosen the name nihonium, derived from the name of the country in the local language, and the The other elements are named after the Russian accompanying symbol Nh.