Chestertonrotating.Com
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

So2 and Wine: a Review
OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW SO2 AND WINE: A REVIEW 1 MARCH 2021 OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW WARNING This document has not been submitted to the step procedure for examining resolutions and cannot in any way be treated as an OIV resolution. Only resolutions adopted by the Member States of the OIV have an official character. This document has been drafted in the framework of Expert Group “Food safety” and revised by other OIV Commissions. This document, drafted and developed on the initiative of the OIV, is a collective expert report. © OIV publications, 1st Edition: March 2021 (Paris, France) ISBN 978-2-85038-022-8 OIV - International Organisation of Vine and Wine 35, rue de Monceau F-75008 Paris - France www.oiv.int 2 MARCH 2021 OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW SCOPE The group of experts « Food safety » of the OIV has worked extensively on the safety assessment of different compounds found in vitivinicultural products. This document aims to gather more specific information on SO2. This document has been prepared taking into consideration the information provided during the different sessions of the group of experts “Food safety” and information provided by Member States. Finally, this document, drafted and developed on the initiative of the OIV, is a collective expert report. This review is based on the help of scientific literature and technical works available until date of publishing. COORDINATOR OIV - International Organisation of Vine and Wine AUTHORS Dr. Creina Stockley (AU) Dr. Angelika Paschke-Kratzin (DE) Pr. -

Sulfite: Here, There, Everywhere
Sulfite: Here, There, Everywhere Max T. Baker, PhD Associate Professor Department of Anesthesia University of Iowa Inadvertent Exposures Combustion of fossil fuels, Air pollutant Large quantities as sulfur dioxide are expelled from volcanos Kilauea on the Big Island Small quantities endogenously formed in mammals from sulfur-containing amino acid metabolism Deliberate Exposures As Preservative- Wine, Beer (dates to Roman times From burning sulfur candles) Fruits and Vegetables (reduce browning, extend shelf-life) Pharmaceuticals1 Reductant - Antioxidant - Antimicrobial What are Sulfites? Oxidized Forms of the Sulfur Atom Sulfur Dioxide, MW = 64, bp = - 10oC (gaseous) Sulfur (IV) - Oxidation state of 4 S = Atomic number 16 – electrons/shell, 2,8,6 Sodium Dioxide Readily Hydrates2 Sulfur Carbon Dioxide Dioxide (irritant) H O H2O 2 Sulfurous Unstable Carbonic low acid species acid pH high pH Bisulfite Bicarbonate anion anion Sulfite Carbonate dianion dianion Forms radical Doesn’t form radical Bisulfite Can Combine with SO2 to form Metabisulfite + excess Bisulfite Metabisulfite (disulfite, pyrosulfite) “Sulfite” usually added to drugs as sodium or potassium salts of: Sulfite, Bisulfite, or Metabisulfite Endogenous to Mammals Small quantities formed from sulfur-containing amino acid metabolism - cysteine, methionine3 + - + H2O + 2H + 2 e Sulfite Sulfate Rapidly detoxified by sulfite oxidase (SOX) to form sulfate – a two electron oxidation, molybdenum dependent Two Confirmed Sulfite Toxicities Neurological abnormalities from genetic sulfite oxidase deficiency3 Allergic reactions from exogenous exposure4 Oral, parenteral, inhalational exposure: dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea to life- threatening anaphylactic and asthmatic reactions “The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people." - FDA Prevalence – 3-10% are sulfite sensitive among asthmatic subjects. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Sulfur Dioxide and Some Sulfites, Bisulfites and Metabisulfites
SULFUR DIOXIDE AND SOME SULFITES, BISULFITES AND METABISULFITES 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms and structural and molecular data Sulfr dioxi Chem. Abstr. Serv Reg. No.: 7446-09-5 Replaced CAS Nos.: 8014-94-6; 12396-99-5; 83008-56-4; 89125-89-3 Chem. Abstr. Name; Sulfur dioxide IUPAC Systematic Name: Sulfur dioxide Synonyms: Sulfurous acid anhydride; sulfurous anhydride; sulfurous oxide; sulfur oxide (S02); sulfur superoxide; sulphur dioxide 0=8=0 S02 MoL. wt: 64.07 Sodium sulfte Chem. Abstr. Serv Reg. No.: 7757-83-7 Altemate CAS No.: 10579-83-6 Replaced CAS No.: 68135-69-3 Chem. Abstr. Name: Sulfurous acid, di sodium salt IUPAC Systematic Name: Sulfurous acid, disodium salt Synonyms: Anhydrous sodium sulfite; disodium sulfite; sodium sulphite o 1/ Na · 0 - 8 - 0 · Na Na2S0J MoL. wt: 126.04 Sodium bisulfe Chem. Abstr. Serv Reg. No.: 7631-90-5 Replaced CAS Nos.: 57414-01-4; 69098-86-8; 89830-27-3; 91829-63-9 Chem. Abstr. Name: Sulfurous acid, monosodium salt IUPAC Systematic Name: Sulfurous acid, monosodium salt -131- 132 lARe MONOGRAPHS VOLUME 54 Synonyms: Hydrogen sulfite sodium; monosodium sulfite; sodium acid sulfite; sodium bisulphite; sodium hydrogen sulfite; sodium sulfite (NaHS03) o Il HO - S - a · Na NaHS03 MoL. wt: 104.06 Sodium metabisulfte Chem. Abstr. Serv Reg. No.: 7681-57-4 Altemate CAS No.: 7757-74-6 Replaced CAS No.: 15771-29-6 Chem. Abstr. Name: Disulfurous acid, disodium salt IUPAC Systematic Name: Pyrosulfurous acid, disodium salt Synonyms: Disodium disulfite; disodium metabisulfite; disodium pyrosulfite; sodium disulfite; sodium metabisulphite; sodium pyrosulfite oIl Il0 Na · 0- S - a - S - a · Na .Na2S20S MoL. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide FIRST EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

List of Chemical Resistance of Materials in Contact with Liquid ANALYSETTE 22 Next & ANALYSETTE 28 Imagesizer LASER PARTICLE SIZER & PARTICLE SIZER
List of chemical resistance of materials in contact with liquid ANALYSETTE 22 NeXT & ANALYSETTE 28 ImageSizer LASER PARTICLE SIZER & PARTICLE SIZER This chart information is based on FRITSCH's knowledge and experience and are intended as a guide and not as a guarantee. We cannot assure the perfect performance of the materials as that depends on many factors as working pressure, pressure picks, fluid, ambient temperature, fluid temperature, concentra‐ tion, duration of exposure, etc… Fritsch GmbH Milling and Sizing Industriestrasse 8 D - 55743 Idar-Oberstein Telephone: +49 (0) 6784/ 70-0 Email: [email protected] Internet: www.fritsch.de Version 04/2020 Index 001 Table of contents Table of contents 1 Materials in contact with liquid.................................................... 4 2 Abbreviations................................................................................. 5 3 List of chemical resistance............................................................. 7 3.1 KEY......................................................................................... 7 3.2 Note....................................................................................... 7 3.3 Table 1.................................................................................... 7 3.4 Table 2.................................................................................. 97 3.5 Table 3................................................................................ 105 - 3 - Materials in contact with liquid 1 Materials in contact with liquid Wet dispersion -

ED300212.Pdf
DOCUMENT RESUME ED 300 212 SE 049 707 AUTHOR Borgford, Christie L.; Summerlin, Lee R. TITLE Chemical Activities. Teacher Edition. INSTITUTION American Chemical Society, Washington, D.C. REPORT NO ISBN-0-8412-1416-6 PUB DATE 88 NOTE 329p.; Drawings may not reproduce well. AVAILABLE FROMAmerican Chemical Society, 1155 Sixteenth Street, NW, Washington, LC 20036 ($19.95). PUB TYPE Guides - Classroom Use - Guides (For Teachers) (052) FDRS PRICE MF01 Plus Postage. PC Nct Available from EDRS. DESCRIPTORS Chemical Reactions; Chemistry; *Demonstrations (Educational); *Educational Experiments; *Experiments; High Schools; Instructional Materials; Investigations; Junior High Schools; *Laboratories; *Laboratory Procedures; Middle Schools; Science Activities; Science Education; Science Instruction; Science Teachers; Secondary Education; *Secondary School Science IDENTIFIERS *ChemCom ABSTRACT This sourcebook for chemical activities is designed to be used as a student laboratory book for both junior and senior high school students. The student's role as-a knowledgeable consumer and informed citizen is stressed. Each activity includes a list of needed materials, procedures, reactions, questions, and notes for the teacher which include background information, teaching tips, and answers to the questions. General areas of consideration include chemistry of matter, chemistry of atoms and molecules, chemical reactions, chemical energy and rates of reaction, chemistry around the house, chemistry and the environment, biochemistry, chemistry of living things, chemistry of foods, chemical detectives, tools and techniques of the chemist, and kitchen chemistry. Included is a cross reference of activities by chemical topics, laboratory skills and by major topics of "Chemistry in the Community" (ChemCom). Also included is a listing of useful resources with addresses as well as several charts of chemistry information. -

CHEMICAL RESISTANCE CHART Chemical Resistance Glove Chart
CHEMICAL RESISTANCE CHART Chemical Resistance Glove Chart P = Poor | F = Fair | G = Good | E = Excellent | X = Not Tested Chemical Latex Nitrile 1,1,1,2-Tetrachloroethane P F P 1,1,1-Trichloroethane P P P 1,1,2-Trichloroethane P P P 1,2,4,5-Tetrachlorobenzene X E P 1,2,4-Trichlorobenzene P F P 1,2-Dichlorobenzene (o-Dichlorobenzene) P P P 1,2-Dichloroethylene P P P 1,2-Propylenoxide P P P 1,3-Dioxane F P P 1,4-Dioxane P P P 1-Nitropropane G P P 2-(Dietylamino)ethanol P E P 2-Chloroethanol X P P 2-Hydroxyethyl acrylate P P P 2-Hydroxyethyl methacrylate (HEMA) X X X 2-Nitropropane P P P 2-Nitrotoluene P P P 3-Bromopropionic acid G F X 4,4-Methylenedianiline (MDA) X X X 5-Fluorouracil X X X Acetaldehyde G P P Acetamide G P P Acetate solvent G F P Acetic acid (glacial acetic acid) 99+ % G F F Acetic acid 30% E G F Acetic acid anhydride G F P Acetone F P P Acetonitrile F F P Acetyl chloride P P P Acetylene G E F Acryl Acid P F P Acrylamide, 30-70% P E F Acrylic Acid G G P Acrylonitrile P P P Adipic acid E E X Allyl alcohol P P P Allylamine P P P Allylchloride (3-Chloroporpene) P P P Aluminium Acetate E G X Aluminium Chloride E E G Aluminium Fluoride G E F Aluminium Hydroxide P E F Aluminium Nitrate E E X Aluminium Potassium Sulfate E E F Aluminium Sulfate E E G Amine G P Ammonia anhdyrous P G G Ammonia gas (cold) E E X Ammonia gas (hot) P P X Ammonia Nitrate X F X 74 Chemical Resistance Glove Chart P = Poor | F = Fair | G = Good | E = Excellent | X = Not Tested Chemical Latex Nitrile Ammonia solution, 30% P E X Ammonium Acetate E E X Ammonium -
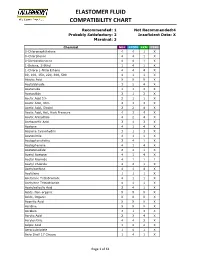
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

The Chemical and Preservative Properties of Sulfur Dioxide Solution for Brining Fruit
The Chemical and Preservative Properties of Sulfur Dioxide Solution for Brining Fruit Circular of Information 629 June 1969 Agricultural Experiment Station, Oregon State University, Corvallis The Chemical and Preservative Properties of Sulfur Dioxide Solution for Brining Fruit C. H. PAYNE, D. V. BEAVERS, and R. F. CAIN Department of Food Science and Technology Sweet cherries and other fruits are preserved in sul- control are given in papers by Waiters et al. (1963) and fur dioxide solutions for manufacture into maraschino, Yang et al. (1966). cocktail, and glace fruit. The sulfur dioxide solution, Sulfur dioxide and calcium are directly related to commonly called brine, may be prepared from liquid brined cherry quality. Improper use of these chemicals sulfur dioxide, sodium bisulfite, or sodium metabisulfite may result in cherries that are soft, poorly bleached, or using alkali or acid to control pH. Calcium salts such as spoiled due to fermentation. It is the purpose of this calcium hydroxide, calcium carbonate, and calcium chlo- circular to show how the basic chemical and preservative ride are added to the brine to prevent cracking and pro- components of brine solutions are afifected during prep- mote firming of the fruit tissue through interaction with aration, storage, and use. pectic materials. Directions for brine preparation and Chemical Properties of Sulfur Dioxide Solutions When sulfur dioxide or materials containing sulfur range of sulfur dioxide concentrations, the relative dis- dioxide (bisulfite or metabisulfite) are dissolved in tribution of the sulfur dioxide ionic forms is constant. water, three types of chemical substances are formed: Within the initial pH range used for brining cher- sulfurous acid (H2SO3),1 bisulfite (HSO3"), and sulfite ries, there is a predominance of calcium bisulfite (SO~). -

Standard X-Ray Diffraction Powder Patterns NATIONAL BUREAU of STANDARDS
NBS MONOGRAPH 25—SECTION 1 9 CO Q U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard X-ray Diffraction Powder Patterns NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essentia! services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration -

Uncorrected Proof
Latin American Applied Research 33:79-86 (2003) EFFECT OF THE ACID-BASE PROPERTIES OF Mg-Al MIXED OXIDES ON THE CATALYST DEACTIVATION DURING ALDOL CONDENSATION REACTIONS V.K. DÍEZ, C.R. APESTEGUÍA and J.I. DI COSIMO* Instituto de Investigaciones en Catálisis y Petroquímica -INCAPE- (UNL-CONICET). Santiago del Estero 2654, (3000) Santa Fe, Argentina, e-mail: [email protected] Abstract−− The effect of chemical composition of have reported that Mg-Al mixed oxides efficiently Mg-Al mixed oxides on both the acid-base properties catalyze the gas-phase self-condensation of acetone to and the deactivation process during the gas phase α,β-unsaturated ketones such as mesityl oxides and self-condensation of acetone was studied. The isophorone (Di Cosimo et al., 1998a). Unfortunately, in activity and selectivity for acetone oligomerization coupling reactions like aldol condensations, basic depended on the catalyst acid-base properties. Mg- catalysts are often deactivated either by the presence of rich catalysts selectively yielded mesityl oxides byproducts such as water in the gas phase or by coke whereas Al-rich MgyAlOx oxides produced mainly build up through secondary side reactions. Deactivation isophorone. The initial deactivation rate, increased has traditionally limited the potential of solid basic linearly with the density of surface basic sites, catalysts to replace environmentally problematic and thereby suggesting that although MgyAlOx oxides corrosive liquid bases. However, few works in the promote the self-condensation of acetone by both literature deal with the deactivation of solid bases under acid- and base-catalyzed mechanisms, the reaction conditions. Studies relating the concerted and deactivation rate would be closely related to the sequential pathways required in the deactivation surface basic properties.