Food Preservative Chemistry: Effects and Side Effects†
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Harmful Effects of Food Preservatives on Human Health Shazia Khanum Mirza1, U.K
Journal of Medicinal Chemistry and Drug Discovery ISSN: 2347-9027 International peer reviewed Journal Special Issue Analytical Chemistry Teacher and Researchers Association National Convention/Seminar Issue 02, Vol. 02, pp. 610-616, 8 January 2017 Available online at www.jmcdd.org To Study The Harmful Effects Of Food Preservatives On Human Health Shazia Khanum Mirza1, U.K. Asema2 And Sayyad Sultan Kasim3. 1 -Research student , Dept of chemistry, Maulana Azad PG & Research centre, Aurangabad. 2-3 -Assist prof. Dept of chemistry,Maulana Azad college Arts sci & com.Aurangabad. ABSTRACT Food chemistry is the study of chemical processes and interactions of all biological and non- biological components. Food additives are chemicals added to foods to keep them fresh or to enhance their color, flavor or texture. They may include food colorings, flavor enhancers or a range of preservatives .The chemical added to a particular food for a particular reason during processing or storage which could affect the characteristics of the food, or become part of the food Preservatives are additives that inhibit the growth of bacteria, yeasts, and molds in foods. Additives and preservatives are used to maintain product consistency and quality, improve or maintain nutritional value, maintain palatability and wholesomeness, provide leavening(yeast), control pH, enhance flavour, or provide colour Some additives have been used for centuries; for example, preserving food by pickling (with vinegar), salting, as with bacon, preserving sweets or using sulfur dioxide as in some wines. Some preservatives are known to be harmful to the human body. Some are classified as carcinogens or cancer causing agents. Keywords : Food , Food additives, colour, flavour , texture, preservatives. -

So2 and Wine: a Review
OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW SO2 AND WINE: A REVIEW 1 MARCH 2021 OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW WARNING This document has not been submitted to the step procedure for examining resolutions and cannot in any way be treated as an OIV resolution. Only resolutions adopted by the Member States of the OIV have an official character. This document has been drafted in the framework of Expert Group “Food safety” and revised by other OIV Commissions. This document, drafted and developed on the initiative of the OIV, is a collective expert report. © OIV publications, 1st Edition: March 2021 (Paris, France) ISBN 978-2-85038-022-8 OIV - International Organisation of Vine and Wine 35, rue de Monceau F-75008 Paris - France www.oiv.int 2 MARCH 2021 OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW SCOPE The group of experts « Food safety » of the OIV has worked extensively on the safety assessment of different compounds found in vitivinicultural products. This document aims to gather more specific information on SO2. This document has been prepared taking into consideration the information provided during the different sessions of the group of experts “Food safety” and information provided by Member States. Finally, this document, drafted and developed on the initiative of the OIV, is a collective expert report. This review is based on the help of scientific literature and technical works available until date of publishing. COORDINATOR OIV - International Organisation of Vine and Wine AUTHORS Dr. Creina Stockley (AU) Dr. Angelika Paschke-Kratzin (DE) Pr. -

Sulfite: Here, There, Everywhere
Sulfite: Here, There, Everywhere Max T. Baker, PhD Associate Professor Department of Anesthesia University of Iowa Inadvertent Exposures Combustion of fossil fuels, Air pollutant Large quantities as sulfur dioxide are expelled from volcanos Kilauea on the Big Island Small quantities endogenously formed in mammals from sulfur-containing amino acid metabolism Deliberate Exposures As Preservative- Wine, Beer (dates to Roman times From burning sulfur candles) Fruits and Vegetables (reduce browning, extend shelf-life) Pharmaceuticals1 Reductant - Antioxidant - Antimicrobial What are Sulfites? Oxidized Forms of the Sulfur Atom Sulfur Dioxide, MW = 64, bp = - 10oC (gaseous) Sulfur (IV) - Oxidation state of 4 S = Atomic number 16 – electrons/shell, 2,8,6 Sodium Dioxide Readily Hydrates2 Sulfur Carbon Dioxide Dioxide (irritant) H O H2O 2 Sulfurous Unstable Carbonic low acid species acid pH high pH Bisulfite Bicarbonate anion anion Sulfite Carbonate dianion dianion Forms radical Doesn’t form radical Bisulfite Can Combine with SO2 to form Metabisulfite + excess Bisulfite Metabisulfite (disulfite, pyrosulfite) “Sulfite” usually added to drugs as sodium or potassium salts of: Sulfite, Bisulfite, or Metabisulfite Endogenous to Mammals Small quantities formed from sulfur-containing amino acid metabolism - cysteine, methionine3 + - + H2O + 2H + 2 e Sulfite Sulfate Rapidly detoxified by sulfite oxidase (SOX) to form sulfate – a two electron oxidation, molybdenum dependent Two Confirmed Sulfite Toxicities Neurological abnormalities from genetic sulfite oxidase deficiency3 Allergic reactions from exogenous exposure4 Oral, parenteral, inhalational exposure: dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea to life- threatening anaphylactic and asthmatic reactions “The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people." - FDA Prevalence – 3-10% are sulfite sensitive among asthmatic subjects. -

Food Preservative Capabilities of Grape (Vitis Vinifera) and Clementine Mandarin (Citrus Reticulata) By-Products Extracts in South Africa
sustainability Article Food Preservative Capabilities of Grape (Vitis vinifera) and Clementine Mandarin (Citrus reticulata) By-products Extracts in South Africa Trust M. Pfukwa 1, Olaniyi A. Fawole 2,3, Marena Manley 1 , Pieter A. Gouws 1, Umezuruike Linus Opara 2,3 and Cletos Mapiye 4,* 1 Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.M.P.); [email protected] (M.M.); [email protected] (P.A.G.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (O.A.F.); [email protected] (U.L.O.) 3 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa 4 Department of Animal Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa * Correspondence: [email protected]; Tel.: +27-21-808-2640 Received: 15 February 2019; Accepted: 19 March 2019; Published: 22 March 2019 Abstract: The drive towards sustainable food systems coupled with increased consumer sophistication have prompted innovation in waste valorization. Grape and citrus processing by-products, abundant in the Mediterranean and tropical regions, respectively, are expanding and are sustainable sources of bioactive phytochemicals that can be used as natural preservatives for foods. Phytochemical composition, antioxidant, and antimicrobial properties of extracts from grape pomace (GPE), seeds (GSE), and clementine mandarin peel and pulp (MPE) grown in South Africa were analyzed. -

The Effect of Preservatives and Flavour Additive on the Production of Oxygen-Free Radicals by Isolated Human Neutrophils
International Journal of Nutrition and Food Sciences 2014; 3(3): 210-215 Published online May 30, 2014 (http://www.sciencepublishinggroup.com/j/ijnfs) doi: 10.11648/j.ijnfs.20140303.23 The effect of preservatives and flavour additive on the production of oxygen-free radicals by isolated human neutrophils E. Al-Shammari 1, R. Bano 1, * , S. Khan 1, I. Shankity 2 1Department of Clinical Nutrition, College of Applied Medical Sciences, University of Hail, Hail, Ksa 2Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, University of Hail, Hail, Ksa Email address: [email protected] (R. Bano) To cite this article: E. Al-Shammari, R. Bano, S. Khan, I. Shankity. The Effect of Preservatives and Flavour Additive on the Production of Oxygen-Free Radicals by Isolated Human Neutrophils. International Journal of Nutrition and Food Sciences. Vol. 3, No. 3, 2014, pp. 210-215. doi: 10.11648/j.ijnfs.20140303.23 Abstract: Introduction: Researchers have recently described a relationship between food additives and immune system responses. A decline in immune response has also been observed in people who are in contact with food additives, such as preservatives and flavours found in different foods. Objectives: The objective of the present study was to investigate the in vitro effect of food additives, such as vanillin, Monosodium L- glutamate, Sodium Benzoate, and potassium nitrate, on the production of oxygen free radicals (OFRs) by isolated human nutrophils. Methods: The study was based on the isolation of neutrophils (polymorphonuclear leukocytes; PMNs) from normal blood to determine the effects of preservatives and flavouring additives on the production of Oxygen Free Radicals by stimulated PMNs. -

Sulfur Dioxide and Some Sulfites, Bisulfites and Metabisulfites
SULFUR DIOXIDE AND SOME SULFITES, BISULFITES AND METABISULFITES 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms and structural and molecular data Sulfr dioxi Chem. Abstr. Serv Reg. No.: 7446-09-5 Replaced CAS Nos.: 8014-94-6; 12396-99-5; 83008-56-4; 89125-89-3 Chem. Abstr. Name; Sulfur dioxide IUPAC Systematic Name: Sulfur dioxide Synonyms: Sulfurous acid anhydride; sulfurous anhydride; sulfurous oxide; sulfur oxide (S02); sulfur superoxide; sulphur dioxide 0=8=0 S02 MoL. wt: 64.07 Sodium sulfte Chem. Abstr. Serv Reg. No.: 7757-83-7 Altemate CAS No.: 10579-83-6 Replaced CAS No.: 68135-69-3 Chem. Abstr. Name: Sulfurous acid, di sodium salt IUPAC Systematic Name: Sulfurous acid, disodium salt Synonyms: Anhydrous sodium sulfite; disodium sulfite; sodium sulphite o 1/ Na · 0 - 8 - 0 · Na Na2S0J MoL. wt: 126.04 Sodium bisulfe Chem. Abstr. Serv Reg. No.: 7631-90-5 Replaced CAS Nos.: 57414-01-4; 69098-86-8; 89830-27-3; 91829-63-9 Chem. Abstr. Name: Sulfurous acid, monosodium salt IUPAC Systematic Name: Sulfurous acid, monosodium salt -131- 132 lARe MONOGRAPHS VOLUME 54 Synonyms: Hydrogen sulfite sodium; monosodium sulfite; sodium acid sulfite; sodium bisulphite; sodium hydrogen sulfite; sodium sulfite (NaHS03) o Il HO - S - a · Na NaHS03 MoL. wt: 104.06 Sodium metabisulfte Chem. Abstr. Serv Reg. No.: 7681-57-4 Altemate CAS No.: 7757-74-6 Replaced CAS No.: 15771-29-6 Chem. Abstr. Name: Disulfurous acid, disodium salt IUPAC Systematic Name: Pyrosulfurous acid, disodium salt Synonyms: Disodium disulfite; disodium metabisulfite; disodium pyrosulfite; sodium disulfite; sodium metabisulphite; sodium pyrosulfite oIl Il0 Na · 0- S - a - S - a · Na .Na2S20S MoL. -

Formaldehyde
FORMALDEHYDE ____________________Name ____________________________________________DateDate alsocalled…formalin,formicaldehyde,methanal,methylaldehyde,methyleneoxide, oxomethane,oxymethylene,morbicidacid.Formaldehydeisreleasedbythesecommon preservatives: quaternium-15 (Dowicilor1-(3-chloroallyl)-3,5,7-triaza-1-azonia-adamantane- chloride),DMDMhydantoin (Glydantor1,3-dimethylol-5,5-dimethylhydantoin), diazolidinyl urea (GermallII), imidazolidinylurea (Germall,imidurea), 2-bromo-2-nitropropane-1,3-diol (Bronopol),andinindustry trisnitromethane (TrisNitro). Whatisit? Formaldehydeisadisinfectantandpreservative. Wheremightitbefound? Photographicdeveloper Make-upremover,toner Mildewresistantfinish,preservative Moisturizingcream,lotion Embalmingfluid,tissuefixative Make-up,concealer,powder Waterproofglue,adhesive,rubbercement Blush,bronzer Latexemulsions,inksolutions,etchingmaterial Mascara,eyepencil,shadow Water-basedpaint,paintprimer,paintstripper Shampoo,bodycleanser Wax,polish,woodcleaner,waterprooffinish Hairconditioner,tonic,spray Plywood,masonite,particleboard,MDF Hairstylinggel,mousse Asphaltshingles,flooring,fillingagents Sunscreen,sunlesstanner Smokefromwood,coal,kerosene,charcoal Bodypowder,talcumpowder Naturalgascombustion,cigar&cigarettesmoke Mouthwash Aftershave,antiperspirant Howtoavoidit: Bubblebath,liquidsoap Toeliminateexposuretoformaldehyde,checkthe Femininehygienespray,rinse completeingredientlist ofeachproductyouuse. Nailpolish,cuticleremover Checkprescriptionmedicinesandcreamstoo. Fingernailhardener Lookforanyofthenamesabove.Forproducts -

Food Preservative Sorbic Acid Deregulates Hepatic Fatty Acid Metabolism
Volume 28 Issue 2 Article 2 2020 Food preservative sorbic acid deregulates hepatic fatty acid metabolism Follow this and additional works at: https://www.jfda-online.com/journal Part of the Food Science Commons, Medicinal Chemistry and Pharmaceutics Commons, Pharmacology Commons, and the Toxicology Commons This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. Recommended Citation Chen, Chia-Hui; Ho, Sin-Ni; Hu, Po-An; Kou, Yu Ru; and Lee, Tzong-Shyuan (2020) "Food preservative sorbic acid deregulates hepatic fatty acid metabolism," Journal of Food and Drug Analysis: Vol. 28 : Iss. 2 , Article 2. Available at: https://doi.org/10.38212/2224-6614.1055 This Original Article is brought to you for free and open access by Journal of Food and Drug Analysis. It has been accepted for inclusion in Journal of Food and Drug Analysis by an authorized editor of Journal of Food and Drug Analysis. ORIGINAL ARTICLE Food preservative sorbic acid deregulates hepatic fatty acid metabolism Chia-Hui Chen a,1, Sin-Ni Ho b,1, Po-An Hu a,1,YuRuKoub, Tzong-Shyuan Lee a,* a Graduate Institute and Department of Physiology, College of Medicine, National Taiwan University, Taipei, Taiwan b Department of Physiology, School of Medicine, National Yang-Ming University, Taipei, Taiwan Abstract Sorbic acid (SA) is one of the most commonly used food preservatives worldwide. Despite SA having no hepato- toxicity at legal dosages, its effect on hepatic lipid metabolism is still unclear. We investigated the effect of SA on hepatic lipid metabolism and its mechanism of action in C57BL/6 mice. -
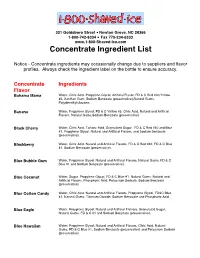
Concentrate Ingredient List
321 Goldsboro Street • Newton Grove, NC 28366 1-800-742-8334 • Fax 770-234-6333 www.1-800-Shaved-Ice.com Concentrate Ingredient List Notice - Concentrate ingredients may occasionally change due to suppliers and flavor profiles. Always check the ingredient label on the bottle to ensure accuracy. Concentrate Ingredients Flavor Bahama Mama Water, Citric Acid, Propylene Glycol, Artificial Flavor, FD & C Red #40,Yellow #5, Xanthan Gum, Sodium Benzoate (preservative),Natural Gums, Polydimethylsilozane. Banana Water, Propylene Glycol, FD & C Yellow #5, Citric Acid, Natural and Artificial Flavors, Natural Gums,Sodium Benzoate (preservative) Black Cherry Water, Citric Acid, Tartaric Acid, Granulated Sugar, FD & C Red #40 and Blue #1, Propylene Glycol, Natural and Artificial Flavors, and Sodium Benzoate (preservative). Blackberry Water, Citric Acid, Natural and Artificial Flavors, FD & C Red #40, FD & C Blue #1, Sodium Benzoate (preservative). Blue Bubble Gum Water, Propylene Glycol, Natural and Artificial Flavors, Natural Gums, FD & C Blue #1 and Sodium Benzoate (preservative). Blue Coconut Water, Sugar, Propylene Glycol, FD & C Blue #1, Natural Gums, Natural and Artificial Flavors, Phosphoric Acid, Potassium Sorbate, Sodium Benzoate (preservative) Blue Cotton Candy Water, Citric Aicd, Natural and Artificial Flavors, Propylene Glycol, FD&C Blue #1, Natural Gums, Titanium Dioxide, Sodium Benzoate and Phosphoric Acid. Blue Eagle Water, Proyplene Glycol, Natural and Artificial Flalvors, Granulated Sugar, Natural Gums, FD & C #1 and Sodium Benzoate (preservative). Blue Hawaiian Water, Propylene Glycol, Natural and Artificial Flavors, Citric Acid, Natural Gums, FD & C Blue #1, Sodium Benzoate (preservative) and Potassium Sorbate (preservative) Blue Raspberry Water,Citric Acid, Propylene Glycol, Natural and Artificial Flavors and FD & C Blue #1. -

HOW TO: Salt and Preservatives in Organic Food
What should I know about using salt and preservatives in organic food? Salt plays an important role in processed food products both for its flavoring properties and as a preservative. For those reasons both salt and sea salt are allowed in organic products as long as the salt or sea salt does not have any prohibited additives. Here we discuss the use of salt, prohibited additives, and answer common questions. Are additives allowed in the salt I use in my organic products? Though salt and sea salt are allowed in all categories of organic products, anti-caking/free-flow agents and whiteners are generally not allowed in these products. Common anti-caking/free- flow agents that are not allowed include: • Calcium silicate • Ferric ammonium citrate • Sodium ferrocyanide • Magnesium silicate • Magnesium carbonate • Propylene glycol • Aluminum calcium silicate • Sodium aluminosilicate Makers of organic products must be able to show that the salt does not contain any of the above flow agents, anti-caking agents, or any whiteners. The producer may need to contact the manufacturer of the salt to get a statement of what is in the salt, or may be required to locate a salt that is free of whiteners and anti-caking and flow agents. There is one exception to this rule, salt formulated with magnesium carbonate is allowed in products labeled as “made with organic ingredients.” However, using salt formulated with magnesium carbonate is prohibited in products labeled “organic” (products containing at least 95% organic ingredients) or “100% organic”. It is possible that salt could be formulated with an anti-caking or flow agent not on the list above. -

Food Additives
Food additives Department of Chemistry The Open University of Sri Lanka 1 Published by The Open University of Sri Lanka 2014 Food additives Introduction In previous lessons, we considered the chemistry of synthetic polymers, natural polymers and biomolecules such as carbohydrates, amino acids, proteins, fatty acids and lipids. In this lesson, we intend to study pros and cons of food additives. People around the world use natural and artificial food additives particularly to improve the taste and appearance of food without thinking of their effects on their health. Do you have the practice of checking the list of food additives given in the label when you buy food items? Centuries ago and even today people smoke fish/meat and add certain chemicals to them (e.g. salt) to improve their shelf life. Particularly in eastern countries, people add spices and indigenous herbs to food to improve its taste and colour. Some food varieties are seasonal (and abundant during the season) and are not available throughout the year. But preserved food is made available around the year, enabling us to enjoy food in different forms, even in places where it is not available or produced. Consumption of food additives may have certain health risks. Carcinogenesis (i.e. causation of cancers), hyperactivity in children, precipitation of allergies, and migraine are some known health risks associated with food additives. It is high time you knew more about what you eat. Let us examine the definition of a food additive. Food additives can be defined as chemical substances deliberately added to food, directly or indirectly, in known or regulated quantities, for purposes of assisting in the processing of food, preservation of food, or in improving the flavour, texture, or appearance of food. -

Life of Foods
Influence of some physical treatments and natural food preservatives on the diversity of microorganisms in certain Egyptian juices By Mohamed Nagah Zayed Ibrahiem Abu El-Naga B.Sc. (Microbiology - chemistry), Fac. Sci., Al-Azhar Univ., Egypt, 2003. THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN Microbiology Department of Botany & Microbiology Faculty of Science Al-Azhar University Cairo 2009 SUPERVISION SHEET Influence of some physical treatments and natural food preservatives on the diversity of microorganisms in certain Egyptian juices By Mohamed Nagah Zayed Ibrahiem Abu El-Naga B.Sc. (Microbiology - chemistry), Fac. Sci., Al-Azhar Univ., Egypt, 2003. SUPERVISION COMMITTEE Prof. Dr. Backry Mohamed Haroun Prof. of Microbiol., Fac. of Sci., Al-Azhar University. Prof. Dr. Mohie El-Din Zohier El-Fouly Prof. of Microbiol., National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority. Dr. Hala Ahmed Hussein Assistant Prof. of Microbiol., National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority. Acknowledgment Praise and gratitude is to ALLAH SOBHANAHU WATAALA, the God of all creatures, for guiding me to the right way. I wish to express my appreciation to Dr. Backry Mohamed Haroun, Professor of Microbiology, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, for helpful advice, encouragement and for sharing in the supervision. Sincere thanks and deep gratitude to Dr. Mohie El-Din Zohier El-Fouly, Professor of Microbiology, National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority, for helpful advice, encouragement, sharing in the supervision and lively interest he showed during the entire thesis.