Principles of Preservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aerobic and Anaerobic Bacterial and Fungal Degradation of Pyrene: Mechanism Pathway Including Biochemical Reaction and Catabolic Genes
International Journal of Molecular Sciences Review Aerobic and Anaerobic Bacterial and Fungal Degradation of Pyrene: Mechanism Pathway Including Biochemical Reaction and Catabolic Genes Ali Mohamed Elyamine 1,2 , Jie Kan 1, Shanshan Meng 1, Peng Tao 1, Hui Wang 1 and Zhong Hu 1,* 1 Key Laboratory of Resources and Environmental Microbiology, Department of Biology, Shantou University, Shantou 515063, China; [email protected] (A.M.E.); [email protected] (J.K.); [email protected] (S.M.); [email protected] (P.T.); [email protected] (H.W.) 2 Department of Life Science, Faculty of Science and Technology, University of Comoros, Moroni 269, Comoros * Correspondence: [email protected] Abstract: Microbial biodegradation is one of the acceptable technologies to remediate and control the pollution by polycyclic aromatic hydrocarbon (PAH). Several bacteria, fungi, and cyanobacteria strains have been isolated and used for bioremediation purpose. This review paper is intended to provide key information on the various steps and actors involved in the bacterial and fungal aerobic and anaerobic degradation of pyrene, a high molecular weight PAH, including catabolic genes and enzymes, in order to expand our understanding on pyrene degradation. The aerobic degradation pathway by Mycobacterium vanbaalenii PRY-1 and Mycobactetrium sp. KMS and the anaerobic one, by the facultative bacteria anaerobe Pseudomonas sp. JP1 and Klebsiella sp. LZ6 are reviewed and presented, to describe the complete and integrated degradation mechanism pathway of pyrene. The different microbial strains with the ability to degrade pyrene are listed, and the degradation of Citation: Elyamine, A.M.; Kan, J.; pyrene by consortium is also discussed. -

The Harmful Effects of Food Preservatives on Human Health Shazia Khanum Mirza1, U.K
Journal of Medicinal Chemistry and Drug Discovery ISSN: 2347-9027 International peer reviewed Journal Special Issue Analytical Chemistry Teacher and Researchers Association National Convention/Seminar Issue 02, Vol. 02, pp. 610-616, 8 January 2017 Available online at www.jmcdd.org To Study The Harmful Effects Of Food Preservatives On Human Health Shazia Khanum Mirza1, U.K. Asema2 And Sayyad Sultan Kasim3. 1 -Research student , Dept of chemistry, Maulana Azad PG & Research centre, Aurangabad. 2-3 -Assist prof. Dept of chemistry,Maulana Azad college Arts sci & com.Aurangabad. ABSTRACT Food chemistry is the study of chemical processes and interactions of all biological and non- biological components. Food additives are chemicals added to foods to keep them fresh or to enhance their color, flavor or texture. They may include food colorings, flavor enhancers or a range of preservatives .The chemical added to a particular food for a particular reason during processing or storage which could affect the characteristics of the food, or become part of the food Preservatives are additives that inhibit the growth of bacteria, yeasts, and molds in foods. Additives and preservatives are used to maintain product consistency and quality, improve or maintain nutritional value, maintain palatability and wholesomeness, provide leavening(yeast), control pH, enhance flavour, or provide colour Some additives have been used for centuries; for example, preserving food by pickling (with vinegar), salting, as with bacon, preserving sweets or using sulfur dioxide as in some wines. Some preservatives are known to be harmful to the human body. Some are classified as carcinogens or cancer causing agents. Keywords : Food , Food additives, colour, flavour , texture, preservatives. -

The Effect Off Ethylenediamine Tetraacetic Acid 4 the Antimicrobial Properties of Benzoic
University of Nigeria Virtual Library Serial No ISSN: 1118-1028 Author 1 MBAH, Chika J. Author 2 Author 3 Title The Effect of Ethylenediamine Tetraacetic Acid on the Antimicrobial Properties of Benzoic Acid and Cetrimide Keywords Description Pharmaceutical Chemistry Category Pharmaceutical Sciences Publisher Publication Date 1999 Signature * - ' 8 - . 1 I r/ Journal of I PHARMACEUTICAL 1 RESEARCH AND i .DEVELOPMENT Journal of Pharmaceutical Research and Development 4: 1 (1 999) 1 -8 - 1 : The Effect offEthylenediamine Tetraacetic Acid 4 the Antimicrobial Properties of Benzoic ) Acid and Cetrimide C. 0. Esirnonel* M. U. ~dikwu';D.B. Uzuegbu' and 0. P. Udeo 4 'Division of Pharmaceutical Microbiology Department of Pharmz Faculty of Phyackutical Sciences University of Nigeria, Ws I 'Department of ~harmacolo~~and Toxicology, Faculty of Pharmaceut ' . University of Nigeria,fisukka. i I I : I. The effect of ethylenediamine tetraacetic acid (EDTA) on the in-vp antimicrobial activities of cetrimide and benzoic acid was evaluated by the checkerboardland killing curve method. The effect sf EDTA aid benzoic acid was evaluated against an isolate of Pseirdonionas aeruginosa (Ps. 021) which is highly resistant to either of the drugs alone. The effect of EDTA and cetrimide was evaluated against isolates of Aspergillus niger and Candidn albicarls resistant to either of the agents. The results show that in the'presence of EDTA, the bacteriostatic and bactericidal effects of benzoic acid and cetrimide against the test microorgan,isms were greatly enhanced. Checkerboard analy& revealed striking synergy (FIC indices > 1 and negative values of activity indices) between almost all the ratios of EDTA and the antimicrobial agents against the various test microorganisms. -

Benzoic Acid
SAFETY DATA SHEET Creation Date 01-May-2012 Revision Date 23-Jan-2015 Revision Number 2 1. Identification Product Name Benzoic acid Cat No. : A63-500; A65-500; A68-30 Synonyms Benzenecarboxylic acid; Benzenemethanoic acid; Phenylcarboxylic acid; Phenylformic acid; Benzeneformic acid; Carboxybenzene Recommended Use Laboratory chemicals. Uses advised against No Information available Details of the supplier of the safety data sheet Company Emergency Telephone Number Fisher Scientific CHEMTRECÒ, Inside the USA: 800-424-9300 One Reagent Lane CHEMTRECÒ, Outside the USA: 001-703-527-3887 Fair Lawn, NJ 07410 Tel: (201) 796-7100 2. Hazard(s) identification Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Skin Corrosion/irritation Category 2 Serious Eye Damage/Eye Irritation Category 1 Specific target organ toxicity - (repeated exposure) Category 1 Target Organs - Lungs. Label Elements Signal Word Danger Hazard Statements Causes skin irritation Causes serious eye damage Causes damage to organs through prolonged or repeated exposure ______________________________________________________________________________________________ Page 1 / 7 Benzoic acid Revision Date 23-Jan-2015 ______________________________________________________________________________________________ Precautionary Statements Prevention Wash face, hands and any exposed skin thoroughly after handling Wear protective gloves/protective clothing/eye protection/face protection Do not breathe dust/fume/gas/mist/vapors/spray Do not eat, drink or smoke when using this product Response Get medical attention/advice if you feel unwell Skin IF ON SKIN: Wash with plenty of soap and water If skin irritation occurs: Get medical advice/attention Take off contaminated clothing and wash before reuse Eyes IF IN EYES: Rinse cautiously with water for several minutes. -
Microflex Gloves Chemical Compatibility Chart
1 1 1 2 2 3 1 CAUTION (LATEX): This product contains natural rubber 2 CAUTION (NITRILE: MEDICAL GRADE): Components used 3 CAUTION (NITRILE: NON-MEDICAL GRADE)): These latex (latex) which may cause allergic reactions. Safe use in making these gloves may cause allergic reactions in gloves are for non-medical use only. They may NOT be of this glove by or on latex sensitized individuals has not some users. Follow your institution’s policies for use. worn for barrier protection in medical or healthcare been established. applications. Please select other gloves for these applications. Components used in making these gloves may cause allergic reactions in some users. Follow your institution’s policies for use. For single use only. NeoPro® Chemicals NeoPro®EC Ethanol ■NBT Ethanolamine (99%) ■NBT Ether ■2 Ethidium bromide (1%) ■NBT Ethyl acetate ■1 Formaldehyde (37%) ■NBT Formamide ■NBT Gluteraldehyde (50%) ■NBT Test Method Description: The test method uses analytical Guanidine hydrochloride ■NBT equipment to determine the concentration of and the time at which (50% ■0 the challenge chemical permeates through the glove film. The Hydrochloric acid ) liquid challenge chemical is collected in a liquid miscible chemical Isopropanol ■NBT (collection media). Data is collected in three separate cells; each cell Methanol ■NBT is compared to a blank cell which uses the same collection media as both the challenge and Methyl ethyl ketone ■0 collection chemical. Methyl methacrylate (33%) ■0 Cautionary Information: These glove recommendations are offered as a guide and for reference Nitric acid (50%) ■NBT purposes only. The barrier properties of each glove type may be affected by differences in material Periodic acid (50%) ■NBT thickness, chemical concentration, temperature, and length of exposure to chemicals. -

Food Preservative Capabilities of Grape (Vitis Vinifera) and Clementine Mandarin (Citrus Reticulata) By-Products Extracts in South Africa
sustainability Article Food Preservative Capabilities of Grape (Vitis vinifera) and Clementine Mandarin (Citrus reticulata) By-products Extracts in South Africa Trust M. Pfukwa 1, Olaniyi A. Fawole 2,3, Marena Manley 1 , Pieter A. Gouws 1, Umezuruike Linus Opara 2,3 and Cletos Mapiye 4,* 1 Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.M.P.); [email protected] (M.M.); [email protected] (P.A.G.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (O.A.F.); [email protected] (U.L.O.) 3 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa 4 Department of Animal Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa * Correspondence: [email protected]; Tel.: +27-21-808-2640 Received: 15 February 2019; Accepted: 19 March 2019; Published: 22 March 2019 Abstract: The drive towards sustainable food systems coupled with increased consumer sophistication have prompted innovation in waste valorization. Grape and citrus processing by-products, abundant in the Mediterranean and tropical regions, respectively, are expanding and are sustainable sources of bioactive phytochemicals that can be used as natural preservatives for foods. Phytochemical composition, antioxidant, and antimicrobial properties of extracts from grape pomace (GPE), seeds (GSE), and clementine mandarin peel and pulp (MPE) grown in South Africa were analyzed. -

The Effect of Preservatives and Flavour Additive on the Production of Oxygen-Free Radicals by Isolated Human Neutrophils
International Journal of Nutrition and Food Sciences 2014; 3(3): 210-215 Published online May 30, 2014 (http://www.sciencepublishinggroup.com/j/ijnfs) doi: 10.11648/j.ijnfs.20140303.23 The effect of preservatives and flavour additive on the production of oxygen-free radicals by isolated human neutrophils E. Al-Shammari 1, R. Bano 1, * , S. Khan 1, I. Shankity 2 1Department of Clinical Nutrition, College of Applied Medical Sciences, University of Hail, Hail, Ksa 2Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, University of Hail, Hail, Ksa Email address: [email protected] (R. Bano) To cite this article: E. Al-Shammari, R. Bano, S. Khan, I. Shankity. The Effect of Preservatives and Flavour Additive on the Production of Oxygen-Free Radicals by Isolated Human Neutrophils. International Journal of Nutrition and Food Sciences. Vol. 3, No. 3, 2014, pp. 210-215. doi: 10.11648/j.ijnfs.20140303.23 Abstract: Introduction: Researchers have recently described a relationship between food additives and immune system responses. A decline in immune response has also been observed in people who are in contact with food additives, such as preservatives and flavours found in different foods. Objectives: The objective of the present study was to investigate the in vitro effect of food additives, such as vanillin, Monosodium L- glutamate, Sodium Benzoate, and potassium nitrate, on the production of oxygen free radicals (OFRs) by isolated human nutrophils. Methods: The study was based on the isolation of neutrophils (polymorphonuclear leukocytes; PMNs) from normal blood to determine the effects of preservatives and flavouring additives on the production of Oxygen Free Radicals by stimulated PMNs. -

Formaldehyde
FORMALDEHYDE ____________________Name ____________________________________________DateDate alsocalled…formalin,formicaldehyde,methanal,methylaldehyde,methyleneoxide, oxomethane,oxymethylene,morbicidacid.Formaldehydeisreleasedbythesecommon preservatives: quaternium-15 (Dowicilor1-(3-chloroallyl)-3,5,7-triaza-1-azonia-adamantane- chloride),DMDMhydantoin (Glydantor1,3-dimethylol-5,5-dimethylhydantoin), diazolidinyl urea (GermallII), imidazolidinylurea (Germall,imidurea), 2-bromo-2-nitropropane-1,3-diol (Bronopol),andinindustry trisnitromethane (TrisNitro). Whatisit? Formaldehydeisadisinfectantandpreservative. Wheremightitbefound? Photographicdeveloper Make-upremover,toner Mildewresistantfinish,preservative Moisturizingcream,lotion Embalmingfluid,tissuefixative Make-up,concealer,powder Waterproofglue,adhesive,rubbercement Blush,bronzer Latexemulsions,inksolutions,etchingmaterial Mascara,eyepencil,shadow Water-basedpaint,paintprimer,paintstripper Shampoo,bodycleanser Wax,polish,woodcleaner,waterprooffinish Hairconditioner,tonic,spray Plywood,masonite,particleboard,MDF Hairstylinggel,mousse Asphaltshingles,flooring,fillingagents Sunscreen,sunlesstanner Smokefromwood,coal,kerosene,charcoal Bodypowder,talcumpowder Naturalgascombustion,cigar&cigarettesmoke Mouthwash Aftershave,antiperspirant Howtoavoidit: Bubblebath,liquidsoap Toeliminateexposuretoformaldehyde,checkthe Femininehygienespray,rinse completeingredientlist ofeachproductyouuse. Nailpolish,cuticleremover Checkprescriptionmedicinesandcreamstoo. Fingernailhardener Lookforanyofthenamesabove.Forproducts -

Food Preservative Sorbic Acid Deregulates Hepatic Fatty Acid Metabolism
Volume 28 Issue 2 Article 2 2020 Food preservative sorbic acid deregulates hepatic fatty acid metabolism Follow this and additional works at: https://www.jfda-online.com/journal Part of the Food Science Commons, Medicinal Chemistry and Pharmaceutics Commons, Pharmacology Commons, and the Toxicology Commons This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. Recommended Citation Chen, Chia-Hui; Ho, Sin-Ni; Hu, Po-An; Kou, Yu Ru; and Lee, Tzong-Shyuan (2020) "Food preservative sorbic acid deregulates hepatic fatty acid metabolism," Journal of Food and Drug Analysis: Vol. 28 : Iss. 2 , Article 2. Available at: https://doi.org/10.38212/2224-6614.1055 This Original Article is brought to you for free and open access by Journal of Food and Drug Analysis. It has been accepted for inclusion in Journal of Food and Drug Analysis by an authorized editor of Journal of Food and Drug Analysis. ORIGINAL ARTICLE Food preservative sorbic acid deregulates hepatic fatty acid metabolism Chia-Hui Chen a,1, Sin-Ni Ho b,1, Po-An Hu a,1,YuRuKoub, Tzong-Shyuan Lee a,* a Graduate Institute and Department of Physiology, College of Medicine, National Taiwan University, Taipei, Taiwan b Department of Physiology, School of Medicine, National Yang-Ming University, Taipei, Taiwan Abstract Sorbic acid (SA) is one of the most commonly used food preservatives worldwide. Despite SA having no hepato- toxicity at legal dosages, its effect on hepatic lipid metabolism is still unclear. We investigated the effect of SA on hepatic lipid metabolism and its mechanism of action in C57BL/6 mice. -
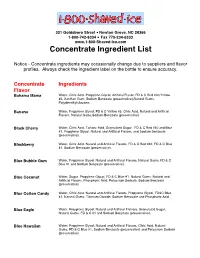
Concentrate Ingredient List
321 Goldsboro Street • Newton Grove, NC 28366 1-800-742-8334 • Fax 770-234-6333 www.1-800-Shaved-Ice.com Concentrate Ingredient List Notice - Concentrate ingredients may occasionally change due to suppliers and flavor profiles. Always check the ingredient label on the bottle to ensure accuracy. Concentrate Ingredients Flavor Bahama Mama Water, Citric Acid, Propylene Glycol, Artificial Flavor, FD & C Red #40,Yellow #5, Xanthan Gum, Sodium Benzoate (preservative),Natural Gums, Polydimethylsilozane. Banana Water, Propylene Glycol, FD & C Yellow #5, Citric Acid, Natural and Artificial Flavors, Natural Gums,Sodium Benzoate (preservative) Black Cherry Water, Citric Acid, Tartaric Acid, Granulated Sugar, FD & C Red #40 and Blue #1, Propylene Glycol, Natural and Artificial Flavors, and Sodium Benzoate (preservative). Blackberry Water, Citric Acid, Natural and Artificial Flavors, FD & C Red #40, FD & C Blue #1, Sodium Benzoate (preservative). Blue Bubble Gum Water, Propylene Glycol, Natural and Artificial Flavors, Natural Gums, FD & C Blue #1 and Sodium Benzoate (preservative). Blue Coconut Water, Sugar, Propylene Glycol, FD & C Blue #1, Natural Gums, Natural and Artificial Flavors, Phosphoric Acid, Potassium Sorbate, Sodium Benzoate (preservative) Blue Cotton Candy Water, Citric Aicd, Natural and Artificial Flavors, Propylene Glycol, FD&C Blue #1, Natural Gums, Titanium Dioxide, Sodium Benzoate and Phosphoric Acid. Blue Eagle Water, Proyplene Glycol, Natural and Artificial Flalvors, Granulated Sugar, Natural Gums, FD & C #1 and Sodium Benzoate (preservative). Blue Hawaiian Water, Propylene Glycol, Natural and Artificial Flavors, Citric Acid, Natural Gums, FD & C Blue #1, Sodium Benzoate (preservative) and Potassium Sorbate (preservative) Blue Raspberry Water,Citric Acid, Propylene Glycol, Natural and Artificial Flavors and FD & C Blue #1. -

Dissolved Organic Matter-Mediated Photodegradation of Anthracene and Pyrene in Water Siyu Zhao, Shuang Xue*, Jinming Zhang, Zhaohong Zhang & Jijun Sun
www.nature.com/scientificreports OPEN Dissolved organic matter-mediated photodegradation of anthracene and pyrene in water Siyu Zhao, Shuang Xue*, Jinming Zhang, Zhaohong Zhang & Jijun Sun Toxicity and transformation process of polycyclic aromatic hydrocarbons (PAHs) is strongly depended on the interaction between PAHs and dissolved organic matters (DOM). In this study, a 125W high- pressure mercury lamp was used to simulate the sunlight experiment to explore the inhibition mechanism of four dissolved organic matters (SRFA, LHA, ESHA, UMRN) on the degradation of anthracene and pyrene in water environment. Results indicated that the photodegradation was the main degradation approach of PAHs, which accorded with the frst-order reaction kinetics equation. The extent of degradation of anthracene and pyrene was 36% and 24%, respectively. DOM infuence mechanism on PAHs varies depending upon its source. SRFA, LHA and ESHA inhibit the photolysis of anthracene, however, except for SRFA, the other three DOM inhibit the photolysis of pyrene. Fluorescence quenching mechanism is the main inhibiting mechanism, and the binding ability of DOM and PAHs is dominantly correlated with its inhibiting efect. FTIR spectroscopies and UV–Visible were used to analyze the main structural changes of DOM binding PAHs. Generally, the stretching vibration of N–H and C–O of polysaccharide carboxylic acid was the key to afect its binding with anthracene and C–O–C in aliphatic ring participated in the complexation of DOM and pyrene. Polycyclic aromatic hydrocarbons (PAHs) are typical persistent organic pollutants with two or more fused ben- zene rings that are widely distributed in multi-media, such as atmosphere, water, sediment, snow, and biota1–4. -

Study of Ethylenediaminetetraacetic Acid (EDTA)
Indian E-Journal of Pharmaceutical Sciences 01[01] 2015 www.asdpub.com/index.php/iejps ISSN: 2454-5244 (Online) Original Article Zero order and area under curve spectrophotometric methods for determination of Aspirin in pharmaceutical formulation Mali Audumbar Digambar*, Hake Gorakhnath, Bathe Ritesh Department of Pharmaceutics, Sahyadri College of Pharmacy, Methwade, Sangola-413307, Solapur, Maharashtra, India *Corresponding Author Abstract Mali Audumbar Digambar Objective: A simple, accurate, precise and specific zero order and area under curve Department of Pharmaceutics, spectrophotometric methods has been developed for determination of Aspirin in its tablet Sahyadri College of Pharmacy, Methwade, dosage form by using methanol as a solvent. Sangola-413307, Solapur, Maharashtra, India Methods: (1) Derivative Spectrophotometric Methods: The amplitudes in the zero order E -mail: [email protected] derivative of the resultant spectra at 224 nm was selected to find out Aspirin in its tablet dosage form by using methanol as a solvent. (2) Area under curve (Area calculation): The proposed area under curve method involves Keywords: measurement of area at selected wavelength ranges. Two wavelength ranges were selected Aspirin, 218-227 nm for estimation of Aspirin. UV visible Result & Discussion: The linearity was found to be 5-25 μg/ml for Aspirin. The mean % Spectrophotometry, recoveries were found to be 99.47% and 100.43% of zero order derivative and area under AUC, curve method of Aspirin. For Repeatability, Intraday precision, Interday precision, % RSD were Method Validation, found to be 0.6416, 0.0046 and 0.9819, 0.8112 for zero order and 0.7257, 0.8731 and 0.7528, Analgesic, Accuracy. 1.7943 for area under curve method respectively.