Altered Profile of Basket Cell Afferent Synapses in Hyperexcitable Dentate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -

The Developmental Genetics of the Cerebellum and the Genetic Bases of Known Cerebellar Disorders
THE DEVELOPMENTAL GENETICS OF THE CEREBELLUM AND THE GENETIC BASES OF KNOWN CEREBELLAR DISORDERS A Review of Developmental Genetics of the Cerebellum and the Genetic Bases of known Cerebellar Disorders Olakada Favour Adamba 17/MHS01/254 Department of Medicine and Health Sciences College of Medicine and Health Sciences Afe Babalola University ANA 303: Neuroanatomy July, 2020 The Developmental Genetics of Cerebellum and the Genetic Bases of known Cerebellar Disorders: A Literature Review Olakada Favour Adamba1 Abstract The internal structure of the cerebellum is an intriguing paradox; its cytoarchitecture is relatively simple compared to the connections between its neurons, which are wired into a complex array of gene expression domains and functional circuits. The genetic research of cerebellar development has provided a great deal of information about the molecular events directing the formation of the cerebellum. The developmental mechanisms that coordinate the establishment of cerebellar structure and circuitry provide a powerful model for understanding how a functional brain develops and its significance in cerebellar disorders and diseases. The cellular makeup of the cerebellum is derived from two primary germinal matrices (the ventricular zone and a specialized germinal matrix called the rhombic lip). Each matrix/zone expresses a specific set of genes that establish the cell lineages within the cerebellar anlage. Then, cohorts of differentiated projection neurons and interneuron progenitors migrate into the developing cerebellum. thereafter, a number of remarkable patterning events occur. Altogether, structural and molecular organisations are thought to support the proper connectivity between incoming afferent projections and their target cells. Key words: Cerebellum, circuitry, genetic, development, disorders. I. Introduction The cerebellum (‘little brain’) resides at the anterior end of the hindbrain and is classically defined by its role in sensory-motor processing (Buckner, 2013). -
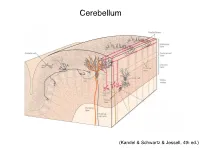
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

An Updated Investigation on the Dromedary Camel Cerebellum (Camelus Dromedarius) with Special Insight Into the Distribution of Calcium‑Binding Proteins Abdelraheim H
www.nature.com/scientificreports OPEN An updated investigation on the dromedary camel cerebellum (Camelus dromedarius) with special insight into the distribution of calcium‑binding proteins Abdelraheim H. Attaai1,3, Ahmed E. Noreldin2, Fatma M. Abdel‑maksoud1* & Manal T. Hussein1,3 Studying the cerebella of diferent animals is important to expand the knowledge about the cerebellum. Studying the camel cerebellum was neglected even though the recent research in the middle east and Asia. Therefore, the present study was designed to achieve a detailed description of the morphology and the cellular organization of the camel cerebellum. Because of the high importance of the calcium ions as a necessary moderator the current work also aimed to investigate the distribution of calcium binding proteins (CaBP) such as calbindin D‑28K (CB), parvalbumin (PV) and calretinin (CR) in diferent cerebellar cells including the non‑traditional neurons. The architecture of camel cerebellum, as diferent mammals, consists of the medulla and three layered‑cortex. According to our observation the cells in the granular layer were not crowded and many spaces were observed. CB expression was the highest by Purkinje cells including their dendritic arborization. In addition to its expression by the inhibitory interneurons (basket, stellate and Golgi neurons), it is also expressed by the excitatory granule cells. PV was expressed by Purkinje cells, including their primary arborization, and by the molecular layer cells. CR immunoreactivity (‑ir) was obvious in almost all cell layers with varying degrees, however a weak or any expression by the Purkinje cells. The molecular layer cells and the Golgi and the non traditional large neurons of the granular layer showed the strongest CR‑ir. -

Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum
712 • The Journal of Neuroscience, January 12, 2011 • 31(2):712–724 Behavioral/Systems/Cognitive Spontaneous Activity Signatures of Morphologically Identified Interneurons in the Vestibulocerebellum Tom J. H. Ruigrok,1 Robert A. Hensbroek,2 and John I. Simpson2 1Department of Neuroscience, Erasmus Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands, and 2Department of Physiology and Neuroscience, New York University School of Medicine, New York, New York 10016 Cerebellar cortical interneurons such as Golgi cells, basket cells, stellate cells, unipolar brush cells, and granule cells play an essential role in the operations of the cerebellum. However, detailed functional studies of the activity of these cells in both anesthetized and behaving animals have been hampered by problems in recognizing their physiological signatures. We have extracellularly recorded the spontane- ous activity of vestibulocerebellar interneurons in ketamine/xylazine-anesthetized rats and subsequently labeled them with Neurobiotin using the juxtacellular technique. After recovery and morphological identification of these cells, they were related to statistical measures of their spontaneous activity. Golgi cells display a somewhat irregular firing pattern with relatively low average frequencies. Unipolar brush cells are characterized by more regular firing at higher rates. Basket and stellate cells are alike in their firing characteristics, which mainly stand out by their irregularity; some of them are set apart by their very slow average rate. The spontaneous activity of interneurons examined in the ketamine/xylazine rabbit fit within this general pattern. In the rabbit, granule cells were identified by the spontaneous occurrence of extremely high-frequency bursts of action potentials, which were also recognized in the rat. -

FS Basket Cells As 2-Stage Nonlinear Integrators
bioRxiv preprint doi: https://doi.org/10.1101/251314; this version posted January 22, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 TITLE 2 Challenging the point neuron dogma: FS basket cells as 2-stage 3 nonlinear integrators 4 AUTHORS 5 Alexandra Tzilivaki1,2 and Panayiota Poirazi1* 6 1 Institute of Molecular Biology and Biotechnology (IMBB), Foundation for Research 7 and Technology Hellas (FORTH), Heraklion, Greece 8 2 Department of Biology, University of Crete, Heraklion, Greece. 9 * Correspondence: Panayiota Poirazi ([email protected]) 10 11 ABSTRACT 12 Interneurons are critical for the proper functioning of neural circuits and are typically 13 considered to act as linear point neurons. However, exciting new findings reveal 14 complex, sub- and/or supralinear computations in the dendrites of various interneuron 15 types. These findings challenge the point neuron dogma and call for a new theory of 16 interneuron arithmetic. Using detailed, biophysically constrained models, we predict 17 that dendrites of FS basket cells in both the hippocampus and mPFC come in two 18 flavors: supralinear, supporting local sodium spikes within large-volume branches and 19 sublinear, in small-volume branches. Synaptic activation of varying sets of these 20 dendrites leads to somatic firing variability that cannot be explained by the point 21 neuron reduction. Instead, a 2-stage Artificial Neural Network (ANN), with both sub- 22 and supralinear hidden nodes, captures the variance. -

''Hairy Baskets'' Associated with Degenerative Purkinje Cell
J Neuropathol Exp Neurol Vol. 69, No. 3 Copyright Ó 2010 by the American Association of Neuropathologists, Inc. March 2010 pp. 262Y271 ORIGINAL ARTICLE ‘‘Hairy Baskets’’ Associated With Degenerative Purkinje Cell Changes in Essential Tremor Cordelia R. Erickson-Davis, BA, Phyllis L. Faust, MD, PhD, Jean-Paul G. Vonsattel, MD, Sachin Gupta, MD, Lawrence S. Honig, MD, PhD, and Elan D. Louis, MD, MSc Downloaded from https://academic.oup.com/jnen/article/69/3/262/2917229 by guest on 30 September 2021 (4). The tremor is usually progressive (5) and produces func- Abstract tional disabilities (6). Recent evidence also suggests an asso- Essential tremor (ET) is one of the most common neurologic dis- ciated increased risk of mortality (7). eases. Increased numbers of torpedoes and Purkinje cell (PC) loss A wealth of clinical data suggests that ET is a disorder have been documented in the brains of patients with ET. We re- of cerebellar dysfunction. Intention (i.e. Bcerebellar[) tremor cently observed a dense and tangled appearance (Bhairiness[) of the of the hands occurs in 44% of ET cases (8). Abnormalities basket cell axonal plexuses that surround PC soma in Bielschowsky in tandem gait and balance have been repeatedly described in preparations of cerebellar cortex in ET brains. Here, we assessed ET patients (9Y11), and ET patients with intention tremor basket cell Bhairiness[ in 37 ET (32 cerebellar ET; 5 Lewy body may also have other cerebellar signs such as dysdiado- variant ET), 21 nondisease control, and 48 disease control brains chokinesia (12). Eye movement abnormalities that indicate using a semiquantitative scale. -
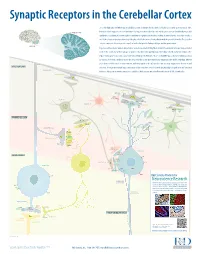
Neuroscience Research CLIMBING FIBERS (Excitatory Input) R&D Systems Off Ers a Wide Range of High Quality Products for Neuroscience Kv4 Research
Synaptic Receptors in the Cerebellar Cortex The cerebellum (Latin for “little brain”) is a highly convoluted structure that lies at the level of the pons on the posterior aspect of the brainstem. It has long been associated with motor activity, and is believed to fi ne-tune muscle action associated with both posture and CEREBELLAR CORTEX equilibrium. In addition, it is now thought to contribute to cognitive and emotional activity. Anatomically, the cerebellum contains a well-defi ned outer cortex plus a distinct set of deeply-embedded neuron cell bodies that form the deep cerebellar nuclei. The erebellarc cortex is composed of three layers: the superfi cial molecular layer, the Purkinje cell layer, and the granular layer. CEREBELLUM In general, extracerebellar neurons project axons, termed mossy and climbing fi bers, to both the cerebellar cortex and deep cerebellar nuclei. In the cortex, mossy fi bers synapse on granule cells, which subsequently project to Purkinje cells, the dominant cell type in the region. Climbing fi bers, by contrast, project directly to Purkinje cells. Purkinje cells are strictly GABAergic, and provide inhibitory output to neurons of the deep cerebellar nuclei. The deep cerebellar nuclei represent the only output structure of the cerebellum. Eff erent axons from cerebellar nuclei neurons transmit inhibitory signals to the inferior olive and excitatory output to the brainstem and MOLECULAR LAYER thalamus. Although the multiple inputs and outputs of the cerebellum are well-defi ned, the physiological signifi cance and functional PARALLEL FIBERS relevance of these microcircuits remains to be established. R&D Systems currently off ers antibodies to all labeled molecules. -

Five Types of Basket Cell in the Hippocampal Dentate Gyrus: a Combined Golgi and Electron Microscopic Study
UC Irvine UC Irvine Previously Published Works Title Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. Permalink https://escholarship.org/uc/item/8q0116vr Journal Journal of neurocytology, 12(4) ISSN 0300-4864 Authors Ribak, CE Seress, L Publication Date 1983-08-01 DOI 10.1007/bf01181525 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Journal of Neurocytology 12, 577-597 (1983) Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study CHARLES E. RIBAK and LASZLO SERESS* Department of Anatomy, University of California, Irvine, California 92717, USA Received 8 September 1982; revised 17 January 1983; accepted 4 February 1983 Summary Five types of basket cell in the hippocampal denate gyrus of rats were analysed with a combined Golgi and electron microscopic method. Light microscopic observations show that the large somata of these different cell types are located either in the granule cell layer or within 30-50 #m of this layer. The sornata of basket cells are pyramidal, horizontal, fusiform or multipolar. Dendrites of basket cells are aspinous or sparsely spinous and are found in all layers of the dentate gyrus. Their axons form an extensive plexus in the granule cell and lower molecular layers. Electron microscopic preparations of Golgi-impregnated, gold-toned basket cells revealed gold-labelled neurons with distinct ultrastructural features. All somata of basket cells displayed an extensive perikaryal cytoplasm with large Nissl bodies and nuclei with infoldings, euchromatin, intranuclear rods and sheets, and large nucleoli. -

Besides Purkinje Cells and Granule Neurons: an Appraisal of the Cell Biology of the Interneurons of the Cerebellar Cortex
Histochem Cell Biol (2008) 130:601–615 DOI 10.1007/s00418-008-0483-y REVIEW Besides Purkinje cells and granule neurons: an appraisal of the cell biology of the interneurons of the cerebellar cortex Karl Schilling · John Oberdick · Ferdinando Rossi · Stephan L. Baader Accepted: 15 July 2008 / Published online: 2 August 2008 © The Author(s) 2008 Abstract Ever since the groundbreaking work of Ramon diversity, and we summarize some recent results that are y Cajal, the cerebellar cortex has been recognized as one of hoped to provide a primer on current understanding of cere- the most regularly structured and wired parts of the brain bellar biology. formed by a rather limited set of distinct cells. Its rather protracted course of development, which persists well into Keywords Interneuron · Basket cell · Stellate cell · postnatal life, the availability of multiple natural mutants, Lugaro cell · Unipolar brush cell · Candelabrum cell · and, more recently, the availability of distinct molecular Development genetic tools to identify and manipulate discrete cell types have suggested the cerebellar cortex as an excellent model to understand the formation and working of the central ner- Introduction vous system. However, the formulation of a unifying model of cerebellar function has so far proven to be a most cantan- “The cerebellar cortex is built from four main types of neu- kerous problem, not least because our understanding of the rons: granule cells, Purkinje cells and two types of inhibi- internal cerebellar cortical circuitry is clearly spotty. Recent tory interneurons, Golgi cells and the stellate/basket cells” research has highlighted the fact that cerebellar cortical (Voogd and Glickstein 1998): To this day, this statement, interneurons are a quite more diverse and heterogeneous or any conceivable permutation or paraphrase of it, is a cor- class of cells than generally appreciated, and have provided nerstone of most descriptions introducing the basic anat- novel insights into the mechanisms that underpin the devel- omy of the cerebellum.