Robust Perisomatic Gabaergic Self- Innervation Inhibits Basket Cells in the Human and Mouse Supragranular Neocortex
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Interactions Among Diameter, Myelination, and the Na/K Pump Affect Axonal Resilience to High-Frequency Spiking
Interactions among diameter, myelination, and the Na/K pump affect axonal resilience to high-frequency spiking Yunliang Zanga,b and Eve Mardera,b,1 aVolen National Center for Complex Systems, Brandeis University, Waltham, MA 02454; and bDepartment of Biology, Brandeis University, Waltham, MA 02454 Contributed by Eve Marder, June 22, 2021 (sent for review March 25, 2021; reviewed by Carmen C. Canavier and Steven A. Prescott) Axons reliably conduct action potentials between neurons and/or (16–21). It remains unknown how these directly correlated pro- other targets. Axons have widely variable diameters and can be cesses, fast spiking and slow Na+ removal, interact in the control myelinated or unmyelinated. Although the effect of these factors of reliable spike generation and propagation. Furthermore, it is on propagation speed is well studied, how they constrain axonal unclear whether reducing Na+-andK+-current overlap can con- resilience to high-frequency spiking is incompletely understood. sistently decrease the rate of Na+ influx and accordingly enhance Maximal firing frequencies range from ∼1Hzto>300 Hz across axonal reliability to propagate high-frequency spikes. Conse- neurons, but the process by which Na/K pumps counteract Na+ quently, by modeling the process of Na+ removal in unmyelinated influx is slow, and the extent to which slow Na+ removal is com- and myelinated axons, we systematically explored the effects of patible with high-frequency spiking is unclear. Modeling the pro- diameter, myelination, and Na/K pump density on spike propagation cess of Na+ removal shows that large-diameter axons are more reliability. resilient to high-frequency spikes than are small-diameter axons, because of their slow Na+ accumulation. -

Energy Expenditure Computation of a Single Bursting Neuron
Cognitive Neurodynamics (2019) 13:75–87 https://doi.org/10.1007/s11571-018-9503-3 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL ARTICLE Energy expenditure computation of a single bursting neuron 2 1,2 2 2 Fengyun Zhu • Rubin Wang • Xiaochuan Pan • Zhenyu Zhu Received: 17 October 2017 / Revised: 22 August 2018 / Accepted: 28 August 2018 / Published online: 3 September 2018 Ó The Author(s) 2018 Abstract Brief bursts of high-frequency spikes are a common firing pattern of neurons. The cellular mechanisms of bursting and its biological significance remain a matter of debate. Focusing on the energy aspect, this paper proposes a neural energy calculation method based on the Chay model of bursting. The flow of ions across the membrane of the bursting neuron with or without current stimulation and its power which contributes to the change of the transmembrane electrical potential energy are analyzed here in detail. We find that during the depolarization of spikes in bursting this power becomes negative, which was also discovered in previous research with another energy model. We also find that the neuron’s energy consumption during bursting is minimal. Especially in the spontaneous state without stimulation, the total energy con- sumption (2.152 9 10-7 J) during 30 s of bursting is very similar to the biological energy consumption (2.468 9 10-7 J) during the generation of a single action potential, as shown in Wang et al. (Neural Plast 2017, 2017a). Our results suggest that this property of low energy consumption could simply be the consequence of the biophysics of generating bursts, which is consistent with the principle of energy minimization. -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Characteristics of Hippocampal Primed Burst Potentiation in Vitro and in the Awake Rat
The Journal of Neuroscience, November 1988, 8(11): 4079-4088 Characteristics of Hippocampal Primed Burst Potentiation in vitro and in the Awake Rat D. M. Diamond, T. V. Dunwiddie, and G. M. Rose Medical Research Service, Veterans Administration Medical Center, and Department of Pharmacology, University of Colorado Health Sciences Center, Denver, Colorado 80220 A pattern of electrical stimulation based on 2 prominent (McNaughton, 1983; Lynch and Baudry, 1984; Farley and Al- physiological features of the hippocampus, complex spike kon, 1985; Byrne, 1987). Of these, a phenomenontermed long- discharge and theta rhythm, was used to induce lasting in- term potentiation (LTP) has received extensive attention be- creases in responses recorded in area CA1 of hippocampal causeit sharesa number of characteristicswith memory. For slices maintained in vitro and from the hippocampus of be- example, LTP has a rapid onset and long duration and is having rats. This effect, termed primed burst (PB) potentia- strengthenedby repetition (Barnes, 1979; Teyler and Discenna, tion, was elicited by as few as 3 stimuli delivered to the 1987). In addition, the decay of LTP is correlated with the time commissural/associational afferents to CAl. The patterns courseof behaviorally assessedforgetting (Barnes, 1979; Barnes of stimulus presentation consisted of a single priming pulse and McNaughton, 1985). The similarity of LTP and memory followed either 140 or 170 msec later by a high-frequency has fostered an extensive characterization of the conditions un- burst of 2-10 pulses; control stimulation composed of un- derlying the initiation of LTP, as well as its biochemical sub- primed high-frequency trains of up to 10 pulses had no en- strates (Lynch and Baudry, 1984; Wigstrijm and Gustafsson, during effect. -

The Logic Behind Neural Control of Breathing Pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C
www.nature.com/scientificreports OPEN The logic behind neural control of breathing pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C. A. Leite3 The respiratory rhythm generator is spectacular in its ability to support a wide range of activities and Received: 10 January 2019 adapt to changing environmental conditions, yet its operating mechanisms remain elusive. We show Accepted: 29 May 2019 how selective control of inspiration and expiration times can be achieved in a new representation of Published: xx xx xxxx the neural system (called a Boolean network). The new framework enables us to predict the behavior of neural networks based on properties of neurons, not their values. Hence, it reveals the logic behind the neural mechanisms that control the breathing pattern. Our network mimics many features seen in the respiratory network such as the transition from a 3-phase to 2-phase to 1-phase rhythm, providing novel insights and new testable predictions. Te mechanism for generating and controlling the breathing pattern by the respiratory neural circuit has been debated for some time1–6. In 1991, an area of the brainstem, the pre Bötzinger Complex (preBötC), was found essential for breathing7. An isolated single PreBötC neuron could generate tonic spiking (a non-interrupted sequence of action potentials), bursting (a repeating pattern that consists of a sequence of action potentials fol- lowed by a time interval with no action potentials) or silence (no action potentials)8. Tese signals are transmit- ted, through other populations of neurons, to spinal motor neurons that activate the respiratory muscle4,6. Te respiratory muscle contracts when it receives a sequence of action potentials from the motor neurons and relaxes when no action potentials arrive9. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

Synaptophysin Regulates Activity-Dependent Synapse Formation in Cultured Hippocampal Neurons
Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons Leila Tarsa and Yukiko Goda* Division of Biology, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0366 Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved November 20, 2001 (received for review October 29, 2001) Synaptophysin is an abundant synaptic vesicle protein without a homogenotypic syp-mutant cultures, however, synapse forma- definite synaptic function. Here, we examined a role for synapto- tion is similar to that observed for wild-type cultures. Interest- physin in synapse formation in mixed genotype micro-island cul- ingly, the decrease in syp-mutant synapse formation is prevented tures of wild-type and synaptophysin-mutant hippocampal neu- when heterogenotypic cultures are grown in the presence of rons. We show that synaptophysin-mutant synapses are poor tetrodotoxin (TTX) or postsynaptic receptor blockers. Our donors of presynaptic terminals in the presence of competing results demonstrate a role for syp in activity-dependent com- wild-type inputs. In homogenotypic cultures, however, mutant petitive synapse formation. neurons display no apparent deficits in synapse formation com- pared with wild-type neurons. The reduced extent of synaptophy- Materials and Methods sin-mutant synapse formation relative to wild-type synapses in Hippocampal Cultures. Primary cultures of dissociated hippocam- mixed genotype cultures is attenuated by blockers of synaptic pal neurons were prepared from late embryonic (E18–19) or transmission. Our findings indicate that synaptophysin plays a newborn (P1) wild-type and syp-mutant mice as described (10). previously unsuspected role in regulating activity-dependent syn- Briefly, dissected hippocampi were incubated for 30 min in 20 apse formation. -
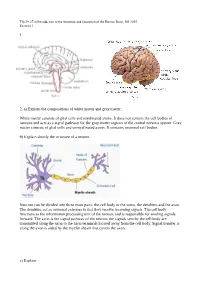
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons.